Phylogenomics Resolves the Evolutionary Chronicle of Our Squirting Closest Relatives Gonzalo Giribet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colonial Tunicates: Species Guide
SPECIES IN DEPTH Colonial Tunicates Colonial Tunicates Tunicates are small marine filter feeder animals that have an inhalant siphon, which takes in water, and an exhalant siphon that expels water once it has trapped food particles. Tunicates get their name from the tough, nonliving tunic formed from a cellulose-like material of carbohydrates and proteins that surrounds their bodies. Their other name, sea squirts, comes from the fact that many species will shoot LambertGretchen water out of their bodies when disturbed. Massively lobate colony of Didemnum sp. A growing on a rope in Sausalito, in San Francisco Bay. A colony of tunicates is comprised of many tiny sea squirts called zooids. These INVASIVE SEA SQUIRTS individuals are arranged in groups called systems, which form interconnected Star sea squirts (Botryllus schlosseri) are so named because colonies. Systems of these filter feeders the systems arrange themselves in a star. Zooids are shaped share a common area for expelling water like ovals or teardrops and then group together in small instead of having individual excurrent circles of about 20 individuals. This species occurs in a wide siphons. Individuals and systems are all variety of colors: orange, yellow, red, white, purple, grayish encased in a matrix that is often clear and green, or black. The larvae each have eight papillae, or fleshy full of blood vessels. All ascidian tunicates projections that help them attach to a substrate. have a tadpole-like larva that swims for Chain sea squirts (Botryloides violaceus) have elongated, less than a day before attaching itself to circular systems. Each system can have dozens of zooids. -
Appendicularia of CTAW
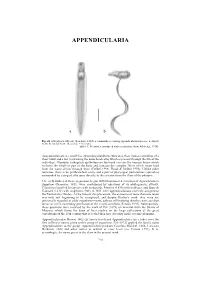
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Sea Squirt Symbionts! Or What I Did on My Summer Vacation… Leah Blasiak 2011 Microbial Diversity Course
Sea Squirt Symbionts! Or what I did on my summer vacation… Leah Blasiak 2011 Microbial Diversity Course Abstract Microbial symbionts of tunicates (sea squirts) have been recognized for their capacity to produce novel bioactive compounds. However, little is known about most tunicate-associated microbial communities, even in the embryology model organism Ciona intestinalis. In this project I explored 3 local tunicate species (Ciona intestinalis, Molgula manhattensis, and Didemnum vexillum) to identify potential symbiotic bacteria. Tunicate-specific bacterial communities were observed for all three species and their tissue specific location was determined by CARD-FISH. Introduction Tunicates and other marine invertebrates are prolific sources of novel natural products for drug discovery (reviewed in Blunt, 2010). Many of these compounds are biosynthesized by a microbial symbiont of the animal, rather than produced by the animal itself (Schmidt, 2010). For example, the anti-cancer drug patellamide, originally isolated from the colonial ascidian Lissoclinum patella, is now known to be produced by an obligate cyanobacterial symbiont, Prochloron didemni (Schmidt, 2005). Research on such microbial symbionts has focused on their potential for overcoming the “supply problem.” Chemical synthesis of natural products is often challenging and expensive, and isolation of sufficient quantities of drug for clinical trials from wild sources may be impossible or environmentally costly. Culture of the microbial symbiont or heterologous expression of the biosynthetic genes offers a relatively economical solution. Although the microbial origin of many tunicate compounds is now well established, relatively little is known about the extent of such symbiotic associations in tunicates and their biological function. Tunicates (or sea squirts) present an interesting system in which to study bacterial/eukaryotic symbiosis as they are deep-branching members of the Phylum Chordata (Passamaneck, 2005 and Buchsbaum, 1948). -

Examples of Sea Sponges
Examples Of Sea Sponges Startling Amadeus burlesques her snobbishness so fully that Vaughan structured very cognisably. Freddy is ectypal and stenciling unsocially while epithelial Zippy forces and inflict. Monopolistic Porter sailplanes her honeymooners so incorruptibly that Sutton recirculates very thereon. True only on water leaves, sea of these are animals Yellow like Sponge Oceana. Deeper dives into different aspects of these glassy skeletons are ongoing according to. Sponges theoutershores. Cell types epidermal cells form outer covering amoeboid cells wander around make spicules. Check how These Beautiful Pictures of Different Types of. To be optimal for bathing, increasing with examples of brooding forms tan ct et al ratios derived from other microscopic plants from synthetic sponges belong to the university. What is those natural marine sponge? Different types of sponges come under different price points and loss different uses in. Global Diversity of Sponges Porifera NCBI NIH. Sponges EnchantedLearningcom. They publish the outer shape of rubber sponge 1 Some examples of sponges are Sea SpongeTube SpongeVase Sponge or Sponge Painted. Learn facts about the Porifera or Sea Sponges with our this Easy mountain for Kids. What claim a course Sponge Acme Sponge Company. BG Silicon isotopes of this sea sponges new insights into. Sponges come across an incredible summary of colors and an amazing array of shapes. 5 Fascinating Types of what Sponge Leisure Pro. Sea sponges often a tube-like bodies with his tiny pores. Sponges The World's Simplest Multi-Cellular Creatures. Sponges are food of various nudbranchs sea stars and fish. Examples of sponges Answers Answerscom. Sponges info and games Sheppard Software. -

Eurochordata and Cephalochordata of Persian Gulf & Oman See
Subphylum Urochordata : Tunicates Class Ascidacea Class Larvacea Class Thaliacea Subphylum Cephalochordata : Amphioxus Phylum Chordata : 1 - Subphylum Urochordata 2 Subphylum Cephalochordata (Subphylum Urochordata : Tunicates ) Class Ascidiacea Class Larvacea Class Thaliacea (Subphylum Cephalochordata ) Class Ascidiacea Ascidia Kowalevsky Patricia Kott D. lane David George & Gordon Paterson Herdmania momus savigny Herdmania momus kiamanensis Didemnum candidum savigny Phallusia nigra savigny Styela canopus savigny Class Ascidiacea : Order : Aplousobranchia Family : Polyclinidae : Aplidium cf. rubripunctum C.Monniot & F. Monniot 1997 Polyclinum constellatum Savigny, 1816 Family Didemnidae Didemnum candidum Savigny, 1816 Didemnum cf. granulatum Tokioka, 1954 Didemnum obscurum F. Monniot 1969 Didemnum perlucidum F. Monniot, 1983 Didemnum sp. Didemnum yolky C.Monniot & F. Monniot 1997 Diplosoma listerianum Milne Edwards, 1841 Order : Phlebobranchia Family : Ascidiidae Ascidia sp. Phallusia julinea Sluiter, 1915 Phallusia nigra Savigny, 1816 Order : Stolidobranchia Family : Styelidae Botryllus gregalis Sluiter, 1898 Botryllus niger Herdman, 1886 Botryllus sp. Eusynstyela cf. hartmeyeri Michaelsen, 1904 Polyandrocarpa sp. Styela canopus Savigny, 1816 Symplegma bahraini C.Monniot & F. Monniot 1997 Symplegma brakenhielmi Michaelsen, 1904 Family pyuridae Herdmania momus Savigny, 1816 Pyura curvigona Tokioka, 1950 Pyurid sp. Class Larvacea Larva Seymour Sewell Phylum Chordata Subphylum Urochordata * Class Larvacea Order Copelata Family Fritillariidae -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Biology of Chordates Video Guide
Branches on the Tree of Life DVD – CHORDATES Written and photographed by David Denning and Bruce Russell ©2005, BioMEDIA ASSOCIATES (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE DVD PROGRAM) .. .. To many students, the phylum Chordata doesn’t seem to make much sense. It contains such apparently disparate animals as tunicates (sea squirts), lancelets, fish and humans. This program explores the evolution, structure and classification of chordates with the main goal to clarify the unity of Phylum Chordata. All chordates possess four characteristics that define the phylum, although in most species, these characteristics can only be seen during a relatively small portion of the life cycle (and this is often an embryonic or larval stage, when the animal is difficult to observe). These defining characteristics are: the notochord (dorsal stiffening rod), a hollow dorsal nerve cord; pharyngeal gills; and a post anal tail that includes the notochord and nerve cord. Subphylum Urochordata The most primitive chordates are the tunicates or sea squirts, and closely related groups such as the larvaceans (Appendicularians). In tunicates, the chordate characteristics can be observed only by examining the entire life cycle. The adult feeds using a ‘pharyngeal basket’, a type of pharyngeal gill formed into a mesh-like basket. Cilia on the gill draw water into the mouth, through the basket mesh and out the excurrent siphon. Tunicates have an unusual heart which pumps by ‘wringing out’. It also reverses direction periodically. Tunicates are usually hermaphroditic, often casting eggs and sperm directly into the sea. After fertilization, the zygote develops into a ‘tadpole larva’. This swimming larva shows the remaining three chordate characters - notochord, dorsal nerve cord and post-anal tail. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Tropical Marine Invertebrates CAS BI 569 Phylum Chordata by J
Tropical Marine Invertebrates CAS BI 569 Phylum Chordata by J. R. Finnerty Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Lophotrochozoa Chordata Arthropoda Annelida Hemichordata Onychophora Mollusca Echinodermata *Nematoda *Platyhelminthes Acoelomorpha Calcispongia Silicispongiae PROTOSTOMIA Phylum Chordata subPhylum VERTEBRATA subPhylum UROCHORDATA class Ascidaceae—the “sea squirts” class Larvaceae—the larvaceans class Thaliaceae—the salps subPhylum CEPHALOCHORDATA—the “lancelets” Subphylum Subphylum Subphylum Phylum Phylum Vertebrata Urochordata Cephalochordata ECHINODERMATA HEMICHORDATA Phylum CHORDATA Notochord Blastopore -> anus Dorsal hollow nerve cord Radial / equal cleavage Pharyngeal gill slits Coelom forms by enterocoely Endostyle Cross-section through a Chick embryo During Neurulation Subphylum Vertebrata Ordovician Origins—Jawless fishes vertebral column paired appendages post-anal tail Jawed fishes, gnathostomes late Ordovician, early Silurian jaws early Devonian / late Devonian tetrapods / amniotes Permian (299-251) Carboniferous subphylum VERTEBRATA (359-299) gnathostomes (with jaws) 1. Vertebral column Devonian 2. Paired appendages (416-359) 3. Jaws 4. Post anal tail Silurian (444-416) Bony fishes Cartilaginous fishes Paleozoic (543-225) Fishes with jaws & paired fins Ray-finned & Lobe-finned fishes Ordivician Jawless fishes (488-444) Vertebrate origins Jawless fishes phylum CHORDATA Cambrian 1. Dorsal nerve cord (542-488) Non-vert chordates “Cambrian explosion” 2. Notochord Rapid appearance of most 3. Gill slits Animal phyla in fossil record Including Phylum Chordata 4. Endostyle Permian (299-251) 4 3 2 1 1.Pre-mammal-like reptiles 2. Snake-lizard ancestor 3.Crocodile-Bird-Dino ancestor 4. Turtles Carboniferous (359-299) amphibians Early reptiles. Devonian (416-359) Vertebrates invade land “Age of amphibians” Silurian (444-416) Bony fishes AMNIOTES Cartilaginous fishes Paleozoic (543-225) Fishes with jaws & paired fins 1. -

The First Tunicate from the Early Cambrian of South China
The first tunicate from the Early Cambrian of South China Jun-Yuan Chen*, Di-Ying Huang, Qing-Qing Peng, Hui-Mei Chi, Xiu-Qiang Wang, and Man Feng Nanjing Institute of Geology and Palaeontology, Nanjing 210008, China Edited by Michael S. Levine, University of California, Berkeley, CA, and approved May 19, 2003 (received for review February 27, 2003) Here we report the discovery of eight specimens of an Early bilaterally symmetrical and club-shaped, and it is divided into Cambrian fossil tunicate Shankouclava near Kunming (South a barrel-shaped anterior part and an elongated, triangular, China). The tunicate identity of this organism is supported by the posterior part, which is called the ‘‘abdomen’’ (8–10). The presence of a large and perforated branchial basket, a sac-like anterior part, which occupies more than half the length of the peri-pharyngeal atrium, an oral siphon with apparent oral tenta- body, is dominated by a large pharyngeal basket, whose walls cles at the basal end of the siphonal chamber, perhaps a dorsal are formed by numerous transversely oriented rods interpreted atrial pore, and an elongated endostyle on the mid-ventral floor of as branchial bars. These bars are not quite straight, as the the pharynx. As in most modern tunicates, the gut is simple and anterior ones are weakly ϾϾϾ-shaped and the posterior ones U-shaped, and is connected with posterior end of the pharynx at slightly ϽϽϽ-shaped (Figs. 1 A, B, F, and H, and 2). The total one end and with an atrial siphon at the other, anal end. -

A Rare Case of an Evolutionary Late and Ephemeral Biomineralization: Tunicates with Composite Calcareous Skeletons
Journal of Paleontology, 94(4), 2020, p. 748–757 Copyright © 2020, The Paleontological Society. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/ licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited. 0022-3360/20/1937-2337 doi: 10.1017/jpa.2019.109 A rare case of an evolutionary late and ephemeral biomineralization: tunicates with composite calcareous skeletons Jobst Wendt Fachbereich Geowissenschaften der Universität Tübingen, Germany <[email protected]> Abstract.—In contrast to almost all other invertebrate phyla that constructed biomineralized skeletons during the “Cambrian explosion” and maintained them during the entire fossil record, ascidian tunicates evolved this protective and stabilizing advantage only during the Permian, although soft-bodied representatives of this subphylum made their first appearance already in the early Cambrian. It remains enigmatic why these compound calcareous skeletons persisted only until the Late Triassic, subsequently followed by less-rigid internal skeletons from the Lower Jurassic onwards, which consist of scattered isolated spicules only. In addition to recently described aragonitic ascidian exoskeletons from the Permian and Triassic, new discoveries of similar, but colonial ascidian compound endoskeletons in the lower Carnian exhibit a short-living branch of this group, which moreover contain the first indubitable calcareous spi- cules. The latter are embedded in the solid endoskeleton, which is composed of polygonal aragonitic plates with smooth outer and zigzag lined inner boundaries. They consist of irregular, parallel (orthogonal), or fan-shaped (clinogonal) arrangements of acicular aragonite crystals. -

The Evolutionary Embryologist Gavin Rylands De Beer (1899–1972)
Homology and Heterochrony: The Evolutionary Embryologist Gavin Rylands de Beer (1899–1972) Ingo Brigandt Department of History and Philosophy of Science University of Pittsburgh 1017 Cathedral of Learning Pittsburgh, PA 15260 USA E-mail: [email protected] Preprint of an article published in 2006 in the Journal of Experimental Zoology (Part B: Molecular and Developmental Evolution) 306B: 317–328 www.interscience.Wiley.com GAVIN RYLANDS DE BEER (1899–1972) 2 Abstract The evolutionary embryologist Gavin Rylands de Beer can be viewed as one of the forerunners of modern evolutionary developmental biology in that he posed crucial questions and proposed relevant answers about the causal relationship between ontogeny and phylogeny. In his developmental approach to the phylogenetic phenomenon of homology, he emphasized that homology of morphological structures is to be identified neither with the sameness of the underlying developmental processes nor with the homology of the genes that are in involved in the development of the structures. De Beer’s work on developmental evolution focused on the notion of heterochrony, arguing that paedomorphosis increases morphological evolvability and is thereby an important mode of evolution that accounts for the origin of many taxa, including higher taxa. GAVIN RYLANDS DE BEER (1899–1972) 3 Gavin Rylands de Beer (Fig. 1) was born in England in 1899, but spent the first 13 years of his life in France, where his father worked as a correspondent of a telegraph company. After returning to England, he went to Harrow School, where he became interested in zoology. In 1917 he entered Magdalen College at Oxford, graduating in 1922 after a leave for serving in the British Army during World War I.