Role of Entropy in Colloidal Self-Assembly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gibbs' Paradox and the Definition of Entropy
Entropy 2008, 10, 15-18 entropy ISSN 1099-4300 °c 2008 by MDPI www.mdpi.org/entropy/ Full Paper Gibbs’ Paradox and the Definition of Entropy Robert H. Swendsen Physics Department, Carnegie Mellon University, Pittsburgh, PA 15213, USA E-Mail: [email protected] Received: 10 December 2007 / Accepted: 14 March 2008 / Published: 20 March 2008 Abstract: Gibbs’ Paradox is shown to arise from an incorrect traditional definition of the entropy that has unfortunately become entrenched in physics textbooks. Among its flaws, the traditional definition predicts a violation of the second law of thermodynamics when applied to colloids. By adopting Boltzmann’s definition of the entropy, the violation of the second law is eliminated, the properties of colloids are correctly predicted, and Gibbs’ Paradox vanishes. Keywords: Gibbs’ Paradox, entropy, extensivity, Boltzmann. 1. Introduction Gibbs’ Paradox [1–3] is based on a traditional definition of the entropy in statistical mechanics found in most textbooks [4–6]. According to this definition, the entropy of a classical system is given by the product of Boltzmann’s constant, k, with the logarithm of a volume in phase space. This is shown in many textbooks to lead to the following equation for the entropy of a classical ideal gas of distinguishable particles, 3 E S (E; V; N) = kN[ ln V + ln + X]; (1) trad 2 N where X is a constant. Most versions of Gibbs’ Paradox, including the one I give in this section, rest on the fact that Eq. 1 is not extensive. There is another version of Gibbs’ Paradox that involves the mixing of two gases, which I will discuss in the third section of this paper. -

Gibbs Paradox: Mixing and Non Mixing Potentials T
Physics Education 1 Jul - Sep 2016 Gibbs paradox: Mixing and non mixing potentials T. P. Suresh 1∗, Lisha Damodaran 1 and K. M. Udayanandan 2 1 School of Pure and Applied Physics Kannur University, Kerala- 673 635, INDIA *[email protected] 2 Nehru Arts and Science College, Kanhangad Kerala- 671 314, INDIA (Submitted 12-03-2016, Revised 13-05-2016) Abstract Entropy of mixing leading to Gibbs paradox is studied for different physical systems with and without potentials. The effect of potentials on the mixing and non mixing character of physical systems is discussed. We hope this article will encourage students to search for new problems which will help them understand statistical mechanics better. 1 Introduction namics of any system (which is the main aim of statistical mechanics) we need to calculate Statistical mechanics is the study of macro- the canonical partition function, and then ob- scopic properties of a system from its mi- tain macro properties like internal energy, en- croscopic description. In the ensemble for- tropy, chemical potential, specific heat, etc. malism introduced by Gibbs[1] there are of the system from the partition function. In three ensembles-micro canonical, canonical this article we make use of the canonical en- and grand canonical ensemble. Since we are semble formalism to study the extensive char- not interested in discussing quantum statis- acter of entropy and then to calculate the tics we will use the canonical ensemble for in- entropy of mixing. It is then used to ex- troducing our ideas. To study the thermody- plain the Gibbs paradox. Most text books Volume 32, Number 3, Article Number: 4. -

Inertial Mass of an Elementary Particle from the Holographic Scenario
Document downloaded from: http://hdl.handle.net/10459.1/62944 The final publication is available at: https://doi.org/10.1142/S0217751X17500439 Copyright (c) World Scientific Publishing, 2017 Inertial mass of an elementary particle from the holographic scenario Jaume Gin´e Departament de Matem`atica, Universitat de Lleida, Catalonia, Spain. E{mail: [email protected] Abstract Various attempts have been made to fully explain the mechanism by which a body has inertial mass. Recently it has been proposed that this mechanism is as follows: when an object accelerates in one direction a dy- namical Rindler event horizon forms in the opposite direction, suppressing Unruh radiation on that side by a Rindler-scale Casimir effect whereas the radiation in the other side is only slightly reduce by a Hubble-scale Casimir effect. This produces a net Unruh radiation pressure force that always op- poses the acceleration, just like inertia, although the masses predicted are twice those expected, see [17]. In a later work an error was corrected so that its prediction improves to within 26% of the Planck mass, see [10]. In this paper the expression of the inertial mass of a elementary particle is derived from the holographic scenario giving the exact value of the mass of a Planck particle when it is applied to a Planck particle. Keywords: inertial mass; Unruh radiation; holographic scenario, Dark matter, Dark energy, cosmology. PACS 98.80.-k - Cosmology PACS 04.62.+v - Quantum fields in curved spacetime PACS 06.30.Dr - Mass and density 1 Introduction The equivalence principle introduced by Einstein in 1907 assumes the com- plete local physical equivalence of a gravitational field and a corresponding non- inertial (accelerated) frame of reference (Einstein was thinking of his famous elevator experiment). -
![Quantization of Black Holes Is One of the Important Issues in Physics [1], and There Has Been No Satisfactory Solution Yet](https://docslib.b-cdn.net/cover/4093/quantization-of-black-holes-is-one-of-the-important-issues-in-physics-1-and-there-has-been-no-satisfactory-solution-yet-554093.webp)
Quantization of Black Holes Is One of the Important Issues in Physics [1], and There Has Been No Satisfactory Solution Yet
Quantization of Black Holes Xiao-Gang He1, 2, 3, 4, ∗ and Bo-Qiang Ma1, 2, 4, † 1School of Physics and State Key Laboratory of Nuclear Physics and Technology, Peking University, Beijing 100871 2Department of Physics and Center for Theoretical Sciences, National Taiwan University, Taipei 10617 3Institute of Particle Physics and Cosmology, Department of Physics, Shanghai JiaoTong University, Shanghai 200240 4Center for High-Energy Physics, Peking University, Beijing 100871 Abstract We show that black holes can be quantized in an intuitive and elegant way with results in agreement with conventional knowledge of black holes by using Bohr’s idea of quantizing the motion of an electron inside the atom in quantum mechanics. We find that properties of black holes can be also derived from an Ansatz of quantized entropy ∆S = 4πk∆R/λ, which was suggested in a previous work to unify the black hole entropy formula and Verlinde’s conjecture to explain gravity as an entropic force. Such an Ansatz also explains gravity as an entropic force from quantum effect. This suggests a way to unify gravity with quantum theory. Several interesting and surprising results of black holes are given from which we predict the existence of primordial black holes ranging from Planck scale both in size and energy to big ones in size but with low energy behaviors. PACS numbers: 04.70.Dy, 03.67.-a, 04.70.-s, 04.90.+e arXiv:1003.2510v3 [hep-th] 5 Apr 2010 ∗Electronic address: [email protected] †Electronic address: [email protected] 1 The quantization of black holes is one of the important issues in physics [1], and there has been no satisfactory solution yet. -

Statistical Mechanics of Entropic Forces: Disassembling a Toy
Home Search Collections Journals About Contact us My IOPscience Statistical mechanics of entropic forces: disassembling a toy This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2010 Eur. J. Phys. 31 1353 (http://iopscience.iop.org/0143-0807/31/6/005) View the table of contents for this issue, or go to the journal homepage for more Download details: IP Address: 132.239.16.142 The article was downloaded on 06/12/2011 at 21:15 Please note that terms and conditions apply. IOP PUBLISHING EUROPEAN JOURNAL OF PHYSICS Eur. J. Phys. 31 (2010) 1353–1367 doi:10.1088/0143-0807/31/6/005 Statistical mechanics of entropic forces: disassembling a toy Igor M Sokolov Institut fur¨ Physik, Humboldt-Universitat¨ zu Berlin, Newtonstraße 15, D-12489 Berlin, Germany E-mail: [email protected] Received 21 June 2010, in final form 9 August 2010 Published 23 September 2010 Online at stacks.iop.org/EJP/31/1353 Abstract The notion of entropic forces often stays mysterious to students, especially to ones coming from outside physics. Although thermodynamics works perfectly in all cases when the notion of entropic force is used, no effort is typically made to explain the mechanical nature of the forces. In this paper we discuss the nature of entropic forces as conditional means of constraint forces in systems where interactions are taken into account as mechanical constraints and discuss several examples of such forces. We moreover demonstrate how these forces appear within the standard formalism of statistical thermodynamics and within the mechanical approach based on the Pope–Ching equation, making evident their connection with the equipartition of energy. -

Gibb's Paradox for Distinguishable
Gibb’s paradox for distinguishable K. M. Udayanandan1, R. K. Sathish1, A. Augustine2 1Department of Physics, Nehru Arts and Science College, Kerala-671 314, India. 2Department of Physics, University of Kannur, Kerala 673 635, India. E-mail: [email protected] (Received 22 March 2014, accepted 17 November 2014) Abstract Boltzmann Correction Factor (BCF) N! is used in micro canonical ensemble and canonical ensemble as a dividing term to avoid Gibbs paradox while finding the number of states and partition function for ideal gas. For harmonic oscillators this factor does not come since they are considered to be distinguishable. We here show that BCF comes twice for harmonic oscillators in grand canonical ensemble for entropy to be extensive in classical statistics. Then we extent this observation for all distinguishable systems. Keywords: Gibbs paradox, harmonic oscillator, distinguishable particles. Resumen El factor de corrección de Boltzmann (BCF) N! se utiliza en ensamble microcanónico y ensamble canónico como un término divisor para evitar la paradoja de Gibbs, mientras se busca el número de estados y la función de partición para un gas ideal. Para osciladores armónicos este factor no viene sin que éstos sean considerados para ser distinguibles. Mostramos aquí que BCF llega dos veces para osciladores armónicos en ensamble gran canónico para que la entropía sea ampliamente usada en la estadística clásica. Luego extendemos esta observación para todos los sistemas distinguibles. Palabras clave: paradoja de Gibbs, oscilador armónico, partículas distinguibles. PACS: 03.65.Ge, 05.20-y ISSN 1870-9095 I. INTRODUCTION Statistical mechanics is a mathematical formalism by which II. CANONICAL ENSEMBLE one can determine the macroscopic properties of a system from the information about its microscopic states. -

Classical Particle Indistinguishability, Precisely
Classical Particle Indistinguishability, Precisely James Wills∗1 1Philosophy, Logic and Scientific Method, London School of Economics Forthcoming in The British Journal for the Philosophy of Science Abstract I present a new perspective on the meaning of indistinguishability of classical particles. This leads to a solution to the problem in statisti- cal mechanics of justifying the inclusion of a factor N! in a probability distribution over the phase space of N indistinguishable classical par- ticles. 1 Introduction Considerations of the identity of objects have long been part of philosophical discussion in the natural sciences and logic. These considerations became particularly pertinent throughout the twentieth century with the develop- ment of quantum physics, widely recognized as having interesting and far- reaching implications concerning the identity, individuality, indiscernibility, indistinguishability1 of the elementary components of our ontology. This discussion continues in both the physics and philosophy literature2. ∗[email protected] 1. This menagerie of terms in the literature is apt to cause confusion, especially as there is no clear consensus on what exactly each of these terms mean. Throughout the rest of the paper, I will be concerned with giving a precise definition of a concept which I will label `indistinguishability', and which, I think, matches with how most other commentators use the word. But I believe it to be a distinct debate whether particles are `identical', `individual', or `indiscernible'. The literature I address in this paper therefore does not, for example, overlap with the debate over the status of Leibniz's PII. 2. See French (2000) for an introduction to this discussion and a comprehensive list of references. -

Lecture 6: Entropy
Matthew Schwartz Statistical Mechanics, Spring 2019 Lecture 6: Entropy 1 Introduction In this lecture, we discuss many ways to think about entropy. The most important and most famous property of entropy is that it never decreases Stot > 0 (1) Here, Stot means the change in entropy of a system plus the change in entropy of the surroundings. This is the second law of thermodynamics that we met in the previous lecture. There's a great quote from Sir Arthur Eddington from 1927 summarizing the importance of the second law: If someone points out to you that your pet theory of the universe is in disagreement with Maxwell's equationsthen so much the worse for Maxwell's equations. If it is found to be contradicted by observationwell these experimentalists do bungle things sometimes. But if your theory is found to be against the second law of ther- modynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation. Another possibly relevant quote, from the introduction to the statistical mechanics book by David Goodstein: Ludwig Boltzmann who spent much of his life studying statistical mechanics, died in 1906, by his own hand. Paul Ehrenfest, carrying on the work, died similarly in 1933. Now it is our turn to study statistical mechanics. There are many ways to dene entropy. All of them are equivalent, although it can be hard to see. In this lecture we will compare and contrast dierent denitions, building up intuition for how to think about entropy in dierent contexts. The original denition of entropy, due to Clausius, was thermodynamic. -

Gibbs Paradox of Entropy of Mixing: Experimental Facts, Its Rejection and the Theoretical Consequences
ELECTRONIC JOURNAL OF THEORETICAL CHEMISTRY, VOL. 1, 135±150 (1996) Gibbs paradox of entropy of mixing: experimental facts, its rejection and the theoretical consequences SHU-KUN LIN Molecular Diversity Preservation International (MDPI), Saengergasse25, CH-4054 Basel, Switzerland and Institute for Organic Chemistry, University of Basel, CH-4056 Basel, Switzerland ([email protected]) SUMMARY Gibbs paradox statement of entropy of mixing has been regarded as the theoretical foundation of statistical mechanics, quantum theory and biophysics. A large number of relevant chemical and physical observations show that the Gibbs paradox statement is false. We also disprove the Gibbs paradox statement through consideration of symmetry, similarity, entropy additivity and the de®ned property of ideal gas. A theory with its basic principles opposing Gibbs paradox statement emerges: entropy of mixing increases continuously with the increase in similarity of the relevant properties. Many outstanding problems, such as the validity of Pauling's resonance theory and the biophysical problem of protein folding and the related hydrophobic effect, etc. can be considered on a new theoretical basis. A new energy transduction mechanism, the deformation, is also brie¯y discussed. KEY WORDS Gibbs paradox; entropy of mixing; mixing of quantum states; symmetry; similarity; distinguishability 1. INTRODUCTION The Gibbs paradox of mixing says that the entropy of mixing decreases discontinuouslywith an increase in similarity, with a zero value for mixing of the most similar or indistinguishable subsystems: The isobaric and isothermal mixing of one mole of an ideal ¯uid A and one mole of a different ideal ¯uid B has the entropy increment 1 (∆S)distinguishable = 2R ln 2 = 11.53 J K− (1) where R is the gas constant, while (∆S)indistinguishable = 0(2) for the mixing of indistinguishable ¯uids [1±10]. -
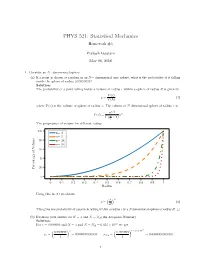
PHYS 521: Statistical Mechanics Homework #4
PHYS 521: Statistical Mechanics Homework #4 Prakash Gautam May 08, 2018 1. Consider an N−dimensional sphere. (a) If a point is chosen at random in an N− dimensional unit sphere, what is the probability of it falling inside the sphere of radius 0:99999999? Solution: The probability of a point falling inside a volume of radius r within a sphere of radius R is given by V (r) p = (1) V (R) where V (x) is the volume of sphere of radius x. The volume of N dimensional sphere of radius x is n=2 (π ) n V (x) = n x Γ 2 + 1 The progression of volume for different radius. 100 n= 2 n= 5 80 n= 24 n= 54 60 40 20 Percentage of Volume 0 0 0:1 0:2 0:3 0:4 0:5 0:6 0:7 0:8 0:9 1 Radius Using this in (1) we obtain ( ) r n p = (2) R This gives the probability of a particle falling within a radius r in a Ndimensional sphere of radius R. □ (b) Evaluate your answer for N = 3 and N = NA(the Avogadro Number) Solution: 23 For r = 0:999999 and N = 3 and N = NA = 6:023 × 10 we get ( ) ( ) × 23 0:999999 3 0:999999 6:023 10 p = = 0:999997000003 p = = 0:0000000000000 3 1 NA 1 1 The probability of a particle falling within the radius nearly 1 in higher two-dimensional sphere is vanishningly small. □ (c) What do these results say about the equivalence of the definitions of entropy in terms of either of the total phase space volume of the volume of outermost energy shell? Solution: Considering a phase space volume bounded by E + ∆ where ∆ ≪ E. -

Colloidal Matter: Packing, Geometry, and Entropy Vinothan N
Colloidal matter: packing, geometry, and entropy Vinothan N. Manoharan Harvard John A. Paulson School of Engineering and Applied Sciences and Department of Physics, Harvard University, Cambridge MA 02138 USA This is the author's version of the work. It is posted here by permission of the AAAS for personal use, not for redistribution. The definitive version was published in Science volume 349 on August 8, 2015. doi: 10.1126/science.1253751 The many dimensions of colloidal matter. The self-assembly of colloids can be controlled by changing the shape, topology, or patchiness of the particles, by introducing attractions between particles, or by constraining them to a curved surface. All of the assembly phenomena illustrated here can be understood from the interplay between entropy and geometrical constraints. Summary of this review article Background: Colloids consist of solid or liquid particles, each about a few hundred nanometers in size, dispersed in a fluid and kept suspended by thermal fluctuations. While natural colloids are the stuff of paint, milk, and glue, synthetic colloids with well-controlled size distributions and interactions are a model system for understanding phase transitions. These colloids can form crystals and other phases of matter seen in atomic and molecular systems, but because the particles are large enough to be seen under an optical microscope, the microscopic mechanisms of phase transitions can be directly observed. Furthermore, their ability to spontaneously form phases that are ordered on the scale of visible wavelengths makes colloids useful building blocks for optical materials such as photonic crystals. Because the interactions between particles can be altered and the effects on structure directly observed, experiments on colloids offer a controlled approach toward understanding and harnessing self-assembly, a fundamental topic in materials science, condensed matter physics, and biophysics. -

Nov/Dec 2020
CERNNovember/December 2020 cerncourier.com COURIERReporting on international high-energy physics WLCOMEE CERN Courier – digital edition ADVANCING Welcome to the digital edition of the November/December 2020 issue of CERN Courier. CAVITY Superconducting radio-frequency (SRF) cavities drive accelerators around the world, TECHNOLOGY transferring energy efficiently from high-power radio waves to beams of charged particles. Behind the march to higher SRF-cavity performance is the TESLA Technology Neutrinos for peace Collaboration (p35), which was established in 1990 to advance technology for a linear Feebly interacting particles electron–positron collider. Though the linear collider envisaged by TESLA is yet ALICE’s dark side to be built (p9), its cavity technology is already established at the European X-Ray Free-Electron Laser at DESY (a cavity string for which graces the cover of this edition) and is being applied at similar broad-user-base facilities in the US and China. Accelerator technology developed for fundamental physics also continues to impact the medical arena. Normal-conducting RF technology developed for the proposed Compact Linear Collider at CERN is now being applied to a first-of-a-kind “FLASH-therapy” facility that uses electrons to destroy deep-seated tumours (p7), while proton beams are being used for novel non-invasive treatments of cardiac arrhythmias (p49). Meanwhile, GANIL’s innovative new SPIRAL2 linac will advance a wide range of applications in nuclear physics (p39). Detector technology also continues to offer unpredictable benefits – a powerful example being the potential for detectors developed to search for sterile neutrinos to replace increasingly outmoded traditional approaches to nuclear nonproliferation (p30).