First Evidence for Long-Term Stasis in Wet-Tropics Land Snail Community Composition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preliminary Malacological Survey of the Site of Community Importance Kamenná Baba (Branisko – Bachureň)
Folia faunistica Slovaca, 2011, 16 (2): 85–89 ISSN 1335-7522 PRELIMINARY MALACOLOGICAL SURVEY OF THE SITE OF COMMUNITY IMPORTANCE KAMENNÁ BABA (BRANISKO – BACHUREŇ) 1 1,2 1 Marek Čiliak & Jozef Šteffek Faculty of Ecology and Environmental Sciences, TU Zvolen, Masarykova 24, 960 53 Zvolen2 [[email protected]] Institute of forest ecology SAS, Štúrova 2, 960 53 Zvolen [[email protected]] Abstract: - Site of community importance (SCI) Kamenná Baba is due to its natu- ral beauties one of the most beautiful and most interesting parts of the eastern Slovakia. Big part of the site represents the same-named National Nature Re- serve. This site was included to the NATURA 2000 network due to the occurrence of several rare species, natural sceneries and biotops. Major part of site is situat ed in the north-eastern part of the Branisko Mts and minor part in the south-west- part of the Bachureň Mts. These mountains are one of the least known areas from the malacological point of view. In septemberClausilia 2009 quantitative dubia carpatica sampling sup plementedPetasina with unidentata individual carpaticacollection was carried out at several sites of the site Kamenná Baba. West Carpathian elementsPerforatella ( dibothrion Brancsik,- 1888, Vestia gulo (Poliński, 1929)) meet the elements with the centre in eastern part ofPupilla Carpathians sterrii ( Pupilla triplicata(M. von Kima kowicz, Vertigo1884), alpestris (E. A. Bielz,Clausilia 1859)) cruciata in this area. Several sozologicaly Balea per- versasignificant species such as (Voith, 1840), (Studer, 1820), Alder, 1838, (Studer, 1820), Key words:(Linnaeus, 1758) are present. Mollusca, SCI Kamenná Baba, species protection, ecoelement, areotyp. INTRODUCTION Vitrea transsylvanica, O orientalis, Macrogastra pli- catula, Cochlodina orthostoma F faustina ( . -

(Mollusca, Gastropoda) of the Bulgarian Part of the Alibotush Mts
Malacologica Bohemoslovaca (2008), 7: 17–20 ISSN 1336-6939 Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. IVAILO KANEV DEDOV Central Laboratory of General Ecology, 2 Gagarin Str., BG-1113 Sofia, Bulgaria, e-mail: [email protected] DEDOV I.K., 2008: Terrestrial gastropods (Mollusca, Gastropoda) of the Bulgarian part of the Alibotush Mts. – Malacologica Bohemoslovaca, 7: 17–20. Online serial at <http://mollusca.sav.sk> 20-Feb-2008. This work presents results of two years collecting efforts within the project “The role of the alpine karst area in Bulgaria as reservoir of species diversity”. It summarizes distribution data of 44 terrestrial gastropods from the Bulgarian part of Alibotush Mts. Twenty-seven species are newly recorded from the Alibotush Mts., 13 were con- firmed, while 4 species, previously known from the literature, were not found. In the gastropod fauna of Alibotush Mts. predominate species from Mediterranean zoogeographic complex. A large part of them is endemic species, and this demonstrates the high conservation value of large limestone areas in respect of terrestrial gastropods. Key words: terrestrial gastropods, distribution, Alibotush Mts., Bulgaria Introduction Locality 6: vill. Katuntsi, Izvorite hut, near hut, open The Alibotush Mts. (other popular names: Kitka, Gotseva ruderal terrain, under bark, 731 m a.s.l., coll. I. Dedov. Planina, Slavjanka) is one of the most interesting large Locality 7: vill. Katuntsi, tufa-gorge near village, 700 m limestone area in Bulgaria (Fig. 1). It occupies the part a.s.l., coll. I. Dedov, N. Simov. of the border region between Bulgaria and Greece with Locality 8: below Livade area, road between Goleshevo maximum elevation 2212 m (Gotsev peak). -

Pulmonata, Helicidae) and the Systematic Position of Cylindrus Obtusus Based on Nuclear and Mitochondrial DNA Marker Sequences
© 2013 The Authors Accepted on 16 September 2013 Journal of Zoological Systematics and Evolutionary Research Published by Blackwell Verlag GmbH J Zoolog Syst Evol Res doi: 10.1111/jzs.12044 Short Communication 1Centre for Ecological and Evolutionary Synthesis (CEES), University of Oslo, Oslo, Norway; 2Central Research Laboratories, Natural History Museum, Vienna, Austria; 33rd Zoological Department, Natural History Museum, Vienna, Austria; 4Department of Integrative Zoology, University of Vienna, Vienna, Austria; 5Department of Zoology, Hungarian Natural History Museum, Budapest, Hungary New data on the phylogeny of Ariantinae (Pulmonata, Helicidae) and the systematic position of Cylindrus obtusus based on nuclear and mitochondrial DNA marker sequences 1 2,4 2,3 3 2 5 LUIS CADAHIA ,JOSEF HARL ,MICHAEL DUDA ,HELMUT SATTMANN ,LUISE KRUCKENHAUSER ,ZOLTAN FEHER , 2,3,4 2,4 LAURA ZOPP and ELISABETH HARING Abstract The phylogenetic relationships among genera of the subfamily Ariantinae (Pulmonata, Helicidae), especially the sister-group relationship of Cylindrus obtusus, were investigated with three mitochondrial (12S rRNA, 16S rRNA, Cytochrome c oxidase subunit I) and two nuclear marker genes (Histone H4 and H3). Within Ariantinae, C. obtusus stands out because of its aberrant cylindrical shell shape. Here, we present phylogenetic trees based on these five marker sequences and discuss the position of C. obtusus and phylogeographical scenarios in comparison with previously published results. Our results provide strong support for the sister-group relationship between Cylindrus and Arianta confirming previous studies and imply that the split between the two genera is quite old. The tree reveals a phylogeographical pattern of Ariantinae with a well-supported clade comprising the Balkan taxa which is the sister group to a clade with individuals from Alpine localities. -

ED45E Rare and Scarce Species Hierarchy.Pdf
104 Species 55 Mollusc 8 Mollusc 334 Species 181 Mollusc 28 Mollusc 44 Species 23 Vascular Plant 14 Flowering Plant 45 Species 23 Vascular Plant 14 Flowering Plant 269 Species 149 Vascular Plant 84 Flowering Plant 13 Species 7 Mollusc 1 Mollusc 42 Species 21 Mollusc 2 Mollusc 43 Species 22 Mollusc 3 Mollusc 59 Species 30 Mollusc 4 Mollusc 59 Species 31 Mollusc 5 Mollusc 68 Species 36 Mollusc 6 Mollusc 81 Species 43 Mollusc 7 Mollusc 105 Species 56 Mollusc 9 Mollusc 117 Species 63 Mollusc 10 Mollusc 118 Species 64 Mollusc 11 Mollusc 119 Species 65 Mollusc 12 Mollusc 124 Species 68 Mollusc 13 Mollusc 125 Species 69 Mollusc 14 Mollusc 145 Species 81 Mollusc 15 Mollusc 150 Species 84 Mollusc 16 Mollusc 151 Species 85 Mollusc 17 Mollusc 152 Species 86 Mollusc 18 Mollusc 158 Species 90 Mollusc 19 Mollusc 184 Species 105 Mollusc 20 Mollusc 185 Species 106 Mollusc 21 Mollusc 186 Species 107 Mollusc 22 Mollusc 191 Species 110 Mollusc 23 Mollusc 245 Species 136 Mollusc 24 Mollusc 267 Species 148 Mollusc 25 Mollusc 270 Species 150 Mollusc 26 Mollusc 333 Species 180 Mollusc 27 Mollusc 347 Species 189 Mollusc 29 Mollusc 349 Species 191 Mollusc 30 Mollusc 365 Species 196 Mollusc 31 Mollusc 376 Species 203 Mollusc 32 Mollusc 377 Species 204 Mollusc 33 Mollusc 378 Species 205 Mollusc 34 Mollusc 379 Species 206 Mollusc 35 Mollusc 404 Species 221 Mollusc 36 Mollusc 414 Species 228 Mollusc 37 Mollusc 415 Species 229 Mollusc 38 Mollusc 416 Species 230 Mollusc 39 Mollusc 417 Species 231 Mollusc 40 Mollusc 418 Species 232 Mollusc 41 Mollusc 419 Species 233 -

Middle and Late Pleistocene Loess-Palaeosol Archives in East Croatia: Multi-Proxy Palaeoecological Studies on Zmajevac and Šarengrad Ii Sequences
Studia Quaternaria, vol. 38, no. 1 (2021): 3–17 DOI: 10.24425/sq.2020.133758 MIDDLE AND LATE PLEISTOCENE LOESS-PALAEOSOL ARCHIVES IN EAST CROATIA: MULTI-PROXY PALAEOECOLOGICAL STUDIES ON ZMAJEVAC AND ŠARENGRAD II SEQUENCES Dávid Molnár1, 2*, László Makó1, 2, Péter Cseh1, 2, Pál Sümegi1, 2, István Fekete3, Lidija Galović4 1 Department of Geology and Paleontology, University of Szeged, H-6722 Szeged, Egyetem u. 2-6, Hungary; e-mail: [email protected] 2 University of Szeged, Interdisciplinary Excellence Centre, Institute of Geography and Earth Sciences, Long Environmental Changes research team, H-6722 Szeged, Egyetem u. 2-6, Hungary; 3 Department of Physical Geography and Geoinformatics, University of Szeged, H-6722 Szeged, Egyetem u. 2-6, Hungary; 4 Croatian Geological Survey, Sachsova 2, 10001 Zagreb, Croatia. * corresponding author Abstract: Multi-proxy palaeoenvironmental analyses on the two loess-palaeosol sequences of Šarengrad II and Zmajevac (Croatia) provided the opportunity to obtain various data on climatic and environmental events that occurred in the southern part of the Carpathian Basin during the past 350,000 years. Palaeoecological horizons were reconstructed using sedimentological data (organic matter and carbonate content, grain-size distribution and magnetic susceptibility) and the dominance-based malacological results (MZs) supported by habitat and richness charts, moreover multi-variate statistics (cluster analysis). The correlation of the reconstructed palaeoecological horizons with global climatic trends (Marine Isotope Stages) determined the main accumulation processes in the examined areas. The palaeoecological analyses revealed specific accumulation conditions at both sequences, fluvial and aeolian environments at Šarengrad and a possible forest refuge at Zmajevac. Key words: palaeoecology, malacology, sedimentology, Šarengrad, Zmajevac, Croatia Manuscript received 17 March 2020, accepted 1 October 2020 INTRODUCTION palaeosol sequences (Zmajevac and Šarengrad II), situated on the right bank of the Danube River (Fig. -

Contribution to the Knowledge of the Terrestrial Gastropods (Mollusca:Gastropoda) from Vrachanska Planina Mountains
Bechev, D. & Georgiev, D. (Eds.), Faunistic diversity of Vrachanski Balkan Nature Park. ZooNotes, Supplemen 3, Plovdiv University Press, Plovdiv, 2016 Contribution to the knowledge of the terrestrial gastropods (Mollusca:Gastropoda) from Vrachanska Planina Mountains IVAILO K. DEDOV, ULRICH E. SCHNEPPAT, FABIA KNECHTLE GLOGGER Abstract. Gastropods fauna from the Vrachanska Planina Mountains (= Vrachanska Planina), Northwest Bulgaria, as well it presents the up to now unpublished results of several research trips of the authors and further collectors in the region. In total 90 terrestrial gastropods species are now known from this mountain area. 78 species were published IURPWKHEHJLQQLQJRIUHVHDUFKLQWKLVDUHDXSWRUHFHQWO\VSHFLHVZHUHFRQÀUPHGZLWK QHZÀQGLQJVDQGVSHFLHVZHUHQRWIRXQGDJDLQZKLOHWKHFRXUVHVRIRXULQYHVWLJDWLRQV JDVWURSRGVSHFLHVDUHQHZO\UHFRUGHGIRUWKHUDQJH Key words: Bulgaria, Vrachanska Planina Mountains, terrestrial gastropods. Introduction )URPWKHEHJLQQLQJRIWKHWKFHQWXU\XQWLOSUHVHQWDXWKRUVKDYHSXEOLVKHG 40 studies concerning the Vrachanska Planina Mts. gastropods fauna. Until the present work 78 terrestrial gastopods species are known to live in this restricted northwestern area of the Stara Planina Mountains Ridge. The Vrachanska Mts. are in shape of an inverted triangle, ZLWKDERXWVLGHOHQJWKVRIDQGNPDQGDEDVHRINPRQO\ ,QWKHFRXUVHRIRXUVWXGLHVVSHFLHVZHUHFRQÀUPHGDQGVXPPDULVHGLQ7DEOH 7KHSUHVHQWZRUNLVDGGLQJQHZVSHFLHVIRUWKHUHJLRQ7KHQHZQXPEHURIVSHFLHVIRU WKH9UDFKDQVND0WVUHSUHVHQWVDERXWRIWKHWHUUHVWULDOJDVWURSRGVVSHFLHVNQRZQ IRU%XOJDULD 0LWRYDQG'HGRY -

Malaco Le Journal Électronique De La Malacologie Continentale Française
MalaCo Le journal électronique de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Paris / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIE / Montpellier / [email protected] Les manuscrits sont à envoyer à : Journal MalaCo Muséum national d’Histoire naturelle Equipe de Malacologie Case Postale 051 55, rue Buffon 75005 Paris Ou par Email à [email protected] MalaCo est téléchargeable gratuitement sur le site : http://www.journal-malaco.fr MalaCo (ISSN 1778-3941) est une publication de l’association Caracol Association Caracol Route de Lodève 34700 Saint-Etienne-de-Gourgas JO Association n° 0034 DE 2003 Déclaration en date du 17 juillet 2003 sous le n° 2569 Journal électronique de la malacologie continentale française MalaCo Septembre 2006 ▪ numéro 3 Au total, 119 espèces et sous-espèces de mollusques, dont quatre strictement endémiques, sont recensées dans les différents habitats du Parc naturel du Mercantour (photos Olivier Gargominy, se reporter aux figures 5, 10 et 17 de l’article d’O. Gargominy & Th. Ripken). Sommaire Page 100 Éditorial Page 101 Actualités Page 102 Librairie Page 103 Brèves & News ▪ Endémisme et extinctions : systématique des Endodontidae (Mollusca, Pulmonata) de Rurutu (Iles Australes, Polynésie française) Gabrielle ZIMMERMANN ▪ The first annual meeting of Task-Force-Limax, Bünder Naturmuseum, Chur, Switzerland, 8-10 September, 2006: presentation, outcomes and abstracts Isabel HYMAN ▪ Collecting and transporting living slugs (Pulmonata: Limacidae) Isabel HYMAN ▪ A List of type specimens of land and freshwater molluscs from France present in the national molluscs collection of the Hebrew University of Jerusalem Henk K. -

Neue Malakologische Befunde Aus Dem Jüngstpleistozänen
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Beiträge zur Paläontologie Jahr/Year: 1996 Band/Volume: 21 Autor(en)/Author(s): Fellner (Frank) Christa, Rabeder Gernot Artikel/Article: Neue malakologische Befunde aus dem jüngstpleistozänen Lößprofil vom Grubgraben bei Kammern (Niederösterreich) 21-31 ©Verein zur Förderung der Paläontologie am Institut für Paläontologie, Geozentrum Wien Beitr. Paläont., 21:21-31, Wien 1996 Neue malakologische Befunde aus dem jüngstpleistozänen Lößprofil vom Grubgraben bei Kammern (Niederösterreich) Malacological news from the Early Pleistocene loess sequence “Grubgraben” near Kammern (Lower Austria) von Christa FRANK* und Gernot RABEDER** FRANK, Ch. & RABEDER, G., 1996. Neue malakologische Befunde aus dem jüngstpleistozänen Lößprofil vom Grubgraben bei Kammern (Niederösterreich). — Beitr. Paläont., 21:21-31, 3 Taf., Wien. Zusammenfassung früherer Grabungen wurden bereits publiziert (MON TET-WHITE, 1990). Die Mollusken-Faunenliste von der Grabungsstelle „Grubgraben“, in MONTET-WHITE (1990) wird zum Diese Studie enthält auch einen Beitrag von HAE- Anlaß einer kritischen Stellungnahme gegen unvoll SAERTS (1990:15-35), in welchem die „mollusc as- ständige Arterfassung und damit unexakte Interpreta semblages“ (det. Prof. J. de Conick und F. Gelaude, Paläontologisches Institut d. Univ. Gent) aufgelistet tion genommen. Der Stellenwert morphologischer Kri terien für eine Präzisierung der Standortsgeschichte sind -

Amaia Caro Aramendia
The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika PhD thesis Vitoria-Gasteiz, 2019 Amaia Caro Aramendia The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika A thesis submitted by Amaia Caro Aramendia for the degree of Doctor of Philosophy, under the supervision of Dr. Benjamín Juan Gómez-Moliner and Dr. María José Madeira University of the Basque Country, Vitoria-Gasteiz, 2019 Zoologia eta Animalia Biologia Zelulen Saila Dpto. Zoología y Biología Celular Animal (cc)2019 AMAIA CARO ARAMENDIA (cc by-nc-nd 4.0) Astiro igo, barraskilotxo Fuji mendia da hau! Kobayashi Issa-ren haikua To the little things that run the world Esker onak Acknowledgements Tesi bat ez da pertsona bakar batena, bidean zehar laguntzen duten pertsona guztiei esker sortutako lana da eta, beraz, lehen orriek haien laguntza eskertzeko izan behar dute: En primer lugar me gustaría agradecer a mis directores, Benjamín Gómez-Moliner y María José Madeira. A Benjamín, por darme la oportunidad de entrar en el grupo de investigación y confiar en que podría realizar esta tesis. Gracias por compartir tus extensos conocimientos y por descubrirme el mundo de la malacología, que sin duda no habría encontrado por mi cuenta y ha resultado de lo más interesante. A Marijo, porque desde el principio y hasta el final has estado siempre ahí para guiarme, animarme y para ayudarme en todo lo que hiciese falta pero, sobre todo, por mostrarme que es posible compaginar este trabajo con una vida fuera de él. -
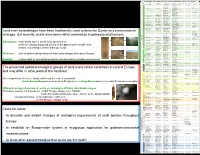
Ecological Groups of Snails – Use and Perspectives
The subdivision of all central European Holocene and Late Glacial land snail species to ecological groups ecological Glacial Early Holocene Middle Holocene Late Holocene (sensu Walker at al 2012) modern immigrants comment group Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acanthinula aculeata Acicula parcelineata Acicula parcelineata Aegopinella epipedostoma one sites Aegopinella nitens Aegopinella nitens Aegopinella nitidula Aegopinella nitidula few sites Aegopinella pura Aegopinella pura Aegopinella pura Aegopinella pura Aegopis verticillus Ecological groups of snails Argna bielzi Argna bielzi Bulgarica cana Bulgarica cana Carpathica calophana Carpathica calophana one site; undated Causa holosericea Causa holosericea Clausilia bidentata no fossil data Clausilia cruciata Clausilia cruciata Clausilia cruciata – use and perspectives Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina laminata Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Cochlodina orthostoma Daudebardia brevipes Daudebardia brevipes Daudebardia rufa Daudebardia rufa Daudebardia rufa Daudebardia rufa Discus perspectivus Discus perspectivus Discus perspectivus 1 2 1 1 ) Lucie Juřičková , Michal Horsák , Jitka Horáčková and Vojen Ložek Discus ruderatus Discus ruderatus Discus ruderatus Discus ruderatus Ena montana Ena montana Ena montana Ena montana forest Eucobresia nivalis Eucobresia nivalis Eucobresia nivalis Faustina faustina Faustina faustina Faustina faustina Faustina faustina Faustina rossmaessleri Faustina -

Draft Carpathian Red List of Forest Habitats
CARPATHIAN RED LIST OF FOREST HABITATS AND SPECIES CARPATHIAN LIST OF INVASIVE ALIEN SPECIES (DRAFT) PUBLISHED BY THE STATE NATURE CONSERVANCY OF THE SLOVAK REPUBLIC 2014 zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 1 227.8.20147.8.2014 222:36:052:36:05 © Štátna ochrana prírody Slovenskej republiky, 2014 Editor: Ján Kadlečík Available from: Štátna ochrana prírody SR Tajovského 28B 974 01 Banská Bystrica Slovakia ISBN 978-80-89310-81-4 Program švajčiarsko-slovenskej spolupráce Swiss-Slovak Cooperation Programme Slovenská republika This publication was elaborated within BioREGIO Carpathians project supported by South East Europe Programme and was fi nanced by a Swiss-Slovak project supported by the Swiss Contribution to the enlarged European Union and Carpathian Wetlands Initiative. zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 2 115.9.20145.9.2014 223:10:123:10:12 Table of contents Draft Red Lists of Threatened Carpathian Habitats and Species and Carpathian List of Invasive Alien Species . 5 Draft Carpathian Red List of Forest Habitats . 20 Red List of Vascular Plants of the Carpathians . 44 Draft Carpathian Red List of Molluscs (Mollusca) . 106 Red List of Spiders (Araneae) of the Carpathian Mts. 118 Draft Red List of Dragonfl ies (Odonata) of the Carpathians . 172 Red List of Grasshoppers, Bush-crickets and Crickets (Orthoptera) of the Carpathian Mountains . 186 Draft Red List of Butterfl ies (Lepidoptera: Papilionoidea) of the Carpathian Mts. 200 Draft Carpathian Red List of Fish and Lamprey Species . 203 Draft Carpathian Red List of Threatened Amphibians (Lissamphibia) . 209 Draft Carpathian Red List of Threatened Reptiles (Reptilia) . 214 Draft Carpathian Red List of Birds (Aves). 217 Draft Carpathian Red List of Threatened Mammals (Mammalia) . -

Fauna from Leaota Mountains - Romania
Muzeul Olteniei Craiova. Oltenia. Studii şi comunicări. Ştiinţele Naturii. Tom. 33, No. 1/2017 ISSN 1454-6914 DIVERSITY OF SOIL MITES (ACARI: MESOSTIGMATA) AND GASTROPODS (GASTROPODA) FAUNA FROM LEAOTA MOUNTAINS - ROMANIA MANU Minodora, LOTREAN Nicolae, CIOBOIU Olivia, POP Oliviu Grigore Abstract. In the period May-September 2016, the diversity of soil mites (Acari: Mesostigmata) and fauna of gastropods (Gastropoda) was investigated in Leaota Mountains. In total 44 transects were investigated, located in 13 types of habitats. Considering the mite fauna, 52 species were identified. The highest value of Shannon index was recorded in natural beech forest, in comparison with the planted spruce forest, where this parameter recorded the lowest value. From the conservative point of view, Pergamasus laetus Juvara-Bals, 1970 was identified as an endemic species for Romania. Another two rare species were signaled: Veigaia propinqua Willmann, 1936 and V. transisalae (Oudemans, 1902). Iphidosoma multiclavatum Willmann, 1953 was signaled for the first time in Romania. Taking into account the gastropod fauna (Gastropoda), 26 species were investigated. The most favorable habitats for these invertebrates were deciduous and spruce forest. One endemic species for Romania was identified: Mastus venerabilis (L. Pfeiffer, 1855). Keywords: abundance, diversity, gastropods, habitat, mites. Rezumat. Diversitatea faunei de acarieni edafici (Acari: Mesostigmata) și de gastropode (Gastropoda) din Munţii Leaota - România. În perioada mai - septembrie 2016, s-a realizat studiul diversităţii faunei de acarieni edafici (Acari: Mesostigmata) şi a gastropodelor (Gastropoda) din munţii Leaota. Au fost investigate 44 de transecte, localizate în 13 tipuri de habitate. Analizând fauna de acarieni, au fost identificate 52 de specii. Cea mai mare valoare a indicelui de diversitate Shannon s-a obţinut în pădurea naturală de fag, în comparaţie cu plantaţia de molid, unde acest parametru a scăzut semnificativ.