WO 2015/028850 Al 5 March 2015 (05.03.2015) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis and Biological Evaluation of Trisindolyl-Cycloalkanes and Bis- Indolyl Naphthalene Small Molecules As Potent Antibacterial and Antifungal Agents
Synthesis and Biological Evaluation of Trisindolyl-Cycloalkanes and Bis- Indolyl Naphthalene Small Molecules as Potent Antibacterial and Antifungal Agents Dissertation Zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) Vorgelegt der Naturwissenschaftlichen Fakultät I Institut für Pharmazie Fachbereich für Pharmazeutische Chemie der Martin-Luther-Universität Halle-Wittenberg von Kaveh Yasrebi Geboren am 09.14.1987 in Teheran/Iran (Islamische Republik) Gutachter: 1. Prof. Dr. Andreas Hilgeroth (Martin-Luther-Universität Halle-Wittenberg, Germany) 2. Prof. Dr. Sibel Süzen (Ankara Üniversitesi, Turkey) 3. Prof. Dr. Michael Lalk (Ernst-Moritz-Arndt-Universität Greifswald, Germany) Halle (Saale), den 21. Juli 2020 Selbstständigkeitserklärung Hiermit erkläre ich gemäß § 5 (2) b der Promotionsordnung der Naturwissenschaftlichen Fakultät I – Institut für Pharmazie der Martin-Luther-Universität Halle-Wittenberg, dass ich die vorliegende Arbeit selbstständig und ohne Benutzung anderer als der angegebenen Hilfsmittel und Quellen angefertigt habe. Alle Stellen, die wörtlich oder sinngemäß aus Veröffentlichungen entnommen sind, habe ich als solche kenntlich gemacht. Ich erkläre ferner, dass diese Arbeit in gleicher oder ähnlicher Form bisher keiner anderen Prüfbehörde zur Erlangung des Doktorgrades vorgelegt wurde. Halle (Saale), den 21. Juli 2020 Kaveh Yasrebi Acknowledgement This study was carried out from June 2015 to July 2017 in the Research Group of Drug Development and Analysis led by Prof. Dr. Andreas Hilgeroth at the Institute of Pharmacy, Martin-Luther-Universität Halle-Wittenberg. I would like to thank all the people for their participation who supported my work in this way and helped me obtain good results. First of all, I would like to express my gratitude to Prof. Dr. Andreas Hilgeroth for providing me with opportunity to carry out my Ph.D. -

(12) United States Patent (10) Patent N0.: US 8,343,962 B2 Kisak Et Al
US008343962B2 (12) United States Patent (10) Patent N0.: US 8,343,962 B2 Kisak et al. (45) Date of Patent: *Jan. 1, 2013 (54) TOPICAL FORMULATION (58) Field of Classi?cation Search ............. .. 514/226.5, 514/334, 420, 557, 567 (75) Inventors: Edward T. Kisak, San Diego, CA (US); See application ?le fOr Complete Search history. John M. NeWsam, La Jolla, CA (US); _ Dominic King-Smith, San Diego, CA (56) References C‘ted (US); Pankaj Karande, Troy, NY (US); Samir Mitragotri, Goleta, CA (US) US' PATENT DOCUMENTS 5,602,183 A 2/1997 Martin et al. (73) Assignee: NuvoResearchOntano (CA) Inc., Mississagua, 6,328,979 2B1 12/2001 Yamashita et a1. 7,001,592 B1 2/2006 Traynor et a1. ( * ) Notice: Subject to any disclaimer, the term of this 7,795,309 B2 9/2010 Kisak eta1~ patent is extended or adjusted under 35 2002/0064524 A1 5/2002 Cevc U.S.C. 154(b) by 212 days. FOREIGN PATENT DOCUMENTS This patent is subject to a terminal dis- W0 WO 2005/009510 2/2005 claimer- OTHER PUBLICATIONS (21) APPI' NO‘, 12/848,792 International Search Report issued on Aug. 8, 2008 in application No. PCT/lB2007/0l983 (corresponding to US 7,795,309). _ Notice ofAlloWance issued on Apr. 29, 2010 by the Examiner in US. (22) Med Aug- 2’ 2010 Appl. No. 12/281,561 (US 7,795,309). _ _ _ Of?ce Action issued on Dec. 30, 2009 by the Examiner in US. Appl. (65) Prior Publication Data No, 12/281,561 (Us 7,795,309), Us 2011/0028460 A1 Feb‘ 3’ 2011 Primary Examiner * Raymond Henley, 111 Related U 5 Application Data (74) Attorney, Agent, or Firm * Foley & Lardner LLP (63) Continuation-in-part of application No. -

Novel Antimicrobial Agents Inhibiting Lipid II Incorporation Into Peptidoglycan Essay MBB
27 -7-2019 Novel antimicrobial agents inhibiting lipid II incorporation into peptidoglycan Essay MBB Mark Nijland S3265978 Supervisor: Prof. Dr. Dirk-Jan Scheffers Molecular Microbiology University of Groningen Content Abstract..............................................................................................................................................2 1.0 Peptidoglycan biosynthesis of bacteria ........................................................................................3 2.0 Novel antimicrobial agents ...........................................................................................................4 2.1 Teixobactin ...............................................................................................................................4 2.2 tridecaptin A1............................................................................................................................7 2.3 Malacidins ................................................................................................................................8 2.4 Humimycins ..............................................................................................................................9 2.5 LysM ........................................................................................................................................ 10 3.0 Concluding remarks .................................................................................................................... 11 4.0 references ................................................................................................................................. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Inclusion and Exclusion Criteria for Each Key Question
Supplemental Table 1: Inclusion and exclusion criteria for each key question Chronic HBV infection in adults ≥ 18 year old (detectable HBsAg in serum for >6 months) Definition of disease Q1 Q2 Q3 Q4 Q5 Q6 Q7 HBV HBV infection with infection and persistent compensated Immunoactive Immunotolerant Seroconverted HBeAg HBV mono-infected viral load cirrhosis with Population chronic HBV chronic HBV from HBeAg to negative population under low level infection infection anti-HBe entecavir or viremia tenofovir (<2000 treatment IU/ml) Adding 2nd Stopped antiviral therapy antiviral drug Interventions and Entecavir compared Antiviral Antiviral therapy compared to continued compared to comparisons to tenofovir therapy therapy continued monotherapy Q1-2: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Intermediate outcomes (if evidence on clinical outcomes is limited or unavailable): HBsAg loss, HBeAg seroconversion and Outcomes HBeAg loss Q3-4: Cirrhosis, decompensated liver disease, HCC, relapse (viral and clinical) and HBsAg loss Q5: Renal function, hypophosphatemia and bone density Q6: Resistance, flare/decompensation and HBeAg loss Q7: Clinical outcomes: Cirrhosis, decompensated liver disease, HCC and death Study design RCT and controlled observational studies Acute HBV infection, children and pregnant women, HIV (+), HCV (+) or HDV (+) persons or other special populations Exclusions such as hemodialysis, transplant, and treatment failure populations. Co treatment with steroids and uncontrolled studies. Supplemental Table 2: Detailed Search Strategy: Ovid Database(s): Embase 1988 to 2014 Week 37, Ovid MEDLINE(R) In-Process & Other Non- Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews - Cochrane Central Register of Controlled Trials August 2014, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to July 2014 Search Strategy: # Searches Results 1 exp Hepatitis B/dt 26410 ("hepatitis B" or "serum hepatitis" or "hippie hepatitis" or "injection hepatitis" or 2 178548 "hepatitis type B").mp. -
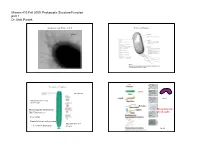
Parsek Micro410 Lecture
Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Organization of the Prokaryotic Cell Prokaryotic Structures fimbriae Size Range of Prokaryotes bacillus See Table 4.1 (rigid) vibrio Nanobacteria 0.05‐0.2 µm (0.14‐0.2 µm) (flexible) Thiomargarita namibiensis Mycoplasma are 700‐750 µm (Fig. 4.2) pleomorphic green alga Nanochlorum eukaryotum Mycoplasma 0.1‐ ~1-2 µm in diameter 0.3 µm Fig. 4.1 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Staining cells for Microscopic observation Cell Arrangements Motility- ~80% of prokaryotes are motile streptococcus Staining properties: Gram Stain staphylococcus Fig. 2.3 Gram Stain (1884) (Bacteria) Gram-negative mixed culture Gram-positive Fig. 2.3 and 2.4 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Functions of the cytoplasmic membrane The phospholipid bi‐layer Fig. 4.9 Fig. 4.4 What is the structure of bacterial phospholipids? Other components of the cytoplasmic membrane Figs. 4.5‐4.6 Microm 410 Fall 2009: Prokaryotic Structure/Function part 1 Dr. Matt Parsek Archaeal membranes can be a lipid monolayer Archaeal phospholipids have an ether linkage Fig. 4.7 Fig. 4.8 Importance of Cell Wall Schematic diagram cell wall • Provides rigidity to cell allowing cell to withstand the large osmotic/ionic Fig. 4.16 changes a bacterium may experience in its environment, and turgor pressure of cytoplasm (conc. of solutes in cytoplasm). Cell lysis • May have a role in shape determination. • Provides a barrier against certain toxic chemical and biological agents. • Site of action of some of the most commonly used antibiotics used to treat bacterial infections (penicillin family). -

(12) United States Patent (10) Patent No.: US 9.468,689 B2 Zeng Et Al
USOO9468689B2 (12) United States Patent (10) Patent No.: US 9.468,689 B2 Zeng et al. (45) Date of Patent: *Oct. 18, 2016 (54) ULTRAFILTRATION CONCENTRATION OF (56) References Cited ALLOTYPE SELECTED ANTIBODES FOR SMALL-VOLUME ADMINISTRATION U.S. PATENT DOCUMENTS 5,429,746 A 7/1995 Shadle et al. (71) Applicant: Immunomedics, Inc., Morris Plains, NJ 5,789,554 A 8/1998 Leung et al. (US) 6,171,586 B1 1/2001 Lam et al. 6,187,287 B1 2/2001 Leung et al. (72) Inventors: Li Zeng, Edison, NJ (US); Rohini 6,252,055 B1 6/2001 Relton Mitra, Brigdewater, NJ (US); Edmund 6,676,924 B2 1/2004 Hansen et al. 6,870,034 B2 3/2005 Breece et al. A. Rossi, Woodland Park, NJ (US); 6,893,639 B2 5/2005 Levy et al. Hans J. Hansen, Picayune, MS (US); 6,991,790 B1 1/2006 Lam et al. David M. Goldenberg, Mendham, NJ 7,038,017 B2 5, 2006 Rinderknecht et al. (US) 7,074,403 B1 7/2006 Goldenberg et al. 7,109,304 B2 9, 2006 Hansen et al. 7,138,496 B2 11/2006 Hua et al. (73) Assignee: Immunomedics, Inc., Morris Plains, NJ 7,151,164 B2 * 12/2006 Hansen et al. ............. 530,387.3 (US) 7,238,785 B2 7/2007 Govindan et al. 7,251,164 B2 7/2007 Okhonin et al. (*) Notice: Subject to any disclaimer, the term of this 7.282,567 B2 10/2007 Goldenberg et al. patent is extended or adjusted under 35 7,300,655 B2 11/2007 Hansen et al. -
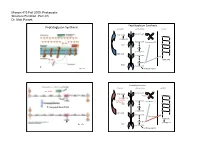
Parsek Lecture #3
Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Peptidoglycan Synthesis Peptidoglycan Synthesis cytoplasm cell membrane cell wall Bactoprenol-P Pi UDP-NAM M G pentapeptide G M Bactoprenol Bactoprenol-P-P P M UMP G P NAM G M pentapeptide M G UDP-NAG Bactoprenol G P NAM‐NAG P NAM-NAG UMP pentapeptide Fig. 6.7a Interbridge peptide Peptidoglycan Synthesis Cross-linking of Peptidoglycan Strands cytoplasm cell membrane cell wall autolysins Bactoprenol-P Pi UDP-NAM Bacitracin M G pentapeptide G D-cycloserine Bactoprenol M (Oxamycin) Bactoprenol-P-P P M UMP G P Transpeptidase (FtsI) NAM G M pentapeptide M G UDP-NAG Bactoprenol G Vancomycin P NAM‐NAG P NAM-NAG pentapeptide UMP Fig. 6.7b pentapeptide Interbridge peptide Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Cross-linking of Peptidoglycan Strands Antibiotic Resistance autolysins • Inactivate antibiotic β-lactamase (penicillinase) Clavulanic acid β-lactams Augmentin and Trimentin (combination of clavulanic acid and transpeptidase amoxicillin or ampicillin respectively) penicillins and cephalosporins lysozyme • Change chemistry of target site • Limit access of the antibiotic to target site Fig. 6.5 Cell Shape Determination • Modifications made to Peptidoglycan: ‐ lysozyme: Protoplasts/spheroplasts ‐ autolysins Bacillus subtilis ‐ endopeptidase Heliobacter pylori • Protein(s) may play a major role ‐ MreB protein Caulobacter crescentus ‐ MreB has homology to actin, a component of the cytoskeleton of eukaryotes. Shape determining protein‐ crescentin Fig. 6.4 Microm 410 Fall 2009: Prokaryotic Structure/Function: Part 2/3 Dr. Matt Parsek Cell Wall Gram-positive Bacteria intermediate filaments in the bacteria Caulobacter crescentus glycerol similar predicted structures of crescentin and intermediate filaments Fig. -

Anticuerpos Monoclonales: Desarrollo Físico Y Perspectivas Terapéuticas
NINA PATRICIA MACHADO ET. AL ARTÍCULO DE REVISIÓN Anticuerpos monoclonales: desarrollo físico y perspectivas terapéuticas Monoclonal antibodies: physical development and therapeutic perspectives NINA PATRICIA MACHADO1, GERMÁN ALBERTO TÉLLEZ2, JOHN CARLOS CASTAÑO2 Resumen Los anticuerpos monoclonales son glucoproteínas Abstract especializadas que hacen parte del sistema inmune, Monoclonal antibodies are specialized glucopro- producidas por las células B, con la capacidad de re- teins that belong to the immune system, produced conocer moléculas específicas (antígenos). Los anti- by the B cells which have the ability to recognize cuerpos monoclonales son herramientas esenciales other molecules (antigens). They are important en el ámbito clínico y biotecnológico, y han probado tools in clinical practice and biotechnology and have ser útiles en el diagnóstico y tratamiento de enfer- been useful in the diagnosis and treatment of medades infecciosas, inmunológicas y neoplá-sicas, infectious, inflammatory, immunological and así como también en el estudio de las interacciones neoplasic diseases, as well as in the study of the patógeno-hospedero y la marcación, detección y cuan- host/patogen interaction, and in the detection and tificación de diversas moléculas. quantification of diverse molecules. Actualmente, la incorporación de las técnicas The incorporation of molecular biology, de biología molecular e ingeniería genética y proteica proteic and genetic engineering have extended the han permitido ampliar el horizonte de la generación production -

Advances and Limitations of Antibody Drug Conjugates for Cancer
biomedicines Review Advances and Limitations of Antibody Drug Conjugates for Cancer Candice Maria Mckertish and Veysel Kayser * Sydney School of Pharmacy, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW 2006, Australia; [email protected] * Correspondence: [email protected]; Tel.: +61-2-9351-3391 Abstract: The popularity of antibody drug conjugates (ADCs) has increased in recent years, mainly due to their unrivalled efficacy and specificity over chemotherapy agents. The success of the ADC is partly based on the stability and successful cleavage of selective linkers for the delivery of the payload. The current research focuses on overcoming intrinsic shortcomings that impact the successful devel- opment of ADCs. This review summarizes marketed and recently approved ADCs, compares the features of various linker designs and payloads commonly used for ADC conjugation, and outlines cancer specific ADCs that are currently in late-stage clinical trials for the treatment of cancer. In addition, it addresses the issues surrounding drug resistance and strategies to overcome resistance, the impact of a narrow therapeutic index on treatment outcomes, the impact of drug–antibody ratio (DAR) and hydrophobicity on ADC clearance and protein aggregation. Keywords: antibody drug conjugates; drug resistance; linkers; payloads; therapeutic index; target specific; ADC clearance; protein aggregation Citation: Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for 1. Introduction Cancer. Biomedicines 2021, 9, 872. Conventional cancer therapy often entails a low therapeutic window and non-specificity https://doi.org/10.3390/ of chemotherapeutic agents that consequently affects normal cells with high mitotic rates biomedicines9080872 and provokes an array of adverse effects, and in some cases leads to drug resistance [1]. -

Pharmacokinetics and Biodistribution of the Anti-Tumor Immunoconjugate, Cantuzumab Mertansine (Huc242-DM1), and Its Two Components in Mice
JPET Fast Forward. Published on November 21, 2003 as DOI: 10.1124/jpet.103.060533 JPET FastThis Forward. article has not Published been copyedited on andNovember formatted. The 21, final 2003 version as mayDOI:10.1124/jpet.103.060533 differ from this version. JPET #60533 Pharmacokinetics and biodistribution of the anti-tumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice Hongsheng Xie, Charlene Audette, Mary Hoffee, John M. Lambert, and Walter A. Blättler ImmunoGen, Inc, 128 Sidney Street, Cambridge, MA 02139 Downloaded from jpet.aspetjournals.org at ASPET Journals on October 2, 2021 1 Copyright 2003 by the American Society for Pharmacology and Experimental Therapeutics. JPET Fast Forward. Published on November 21, 2003 as DOI: 10.1124/jpet.103.060533 This article has not been copyedited and formatted. The final version may differ from this version. JPET #60533 Pharmacokinetics of cantuzumab mertansine in mice Hongsheng Xie ImmunoGen, Inc. 128 Sidney Street Downloaded from Cambridge, MA 02139 Tel.: (617) 995-2500 jpet.aspetjournals.org Fax: (617) 995-2510 e-mail: [email protected] at ASPET Journals on October 2, 2021 Number of text pages: 33 Number of tables: 2 Number of figures: 6 Number of references: 20 Number of words in the abstract: 219 Number of words in the introduction: 587 Number of words in the discussion: 1538 2 JPET Fast Forward. Published on November 21, 2003 as DOI: 10.1124/jpet.103.060533 This article has not been copyedited and formatted. The final version may differ from this version. JPET #60533 Abstract The humanized monoclonal antibody-maytansinoid conjugate, cantuzumab mertansine (huC242-DM1) that contains on average three to four linked drug molecules per antibody molecule was evaluated in CD-1 mice for its pharmacokinetic behavior and tissue distribution and the results were compared with those of the free antibody, huC242. -

Maytenus Ovatus (Schweinf.) an African Medicinal Plant Yielding Potential Anti-Cancer Drugs
Volume 1- Issue 7 : 2017 DOI: 10.26717/BJSTR.2017.01.000571 S Sumesh Kumar. Biomed J Sci & Tech Res ISSN: 2574-1241 Review Article Open Access Maytenus ovatus (schweinf.) An African Medicinal Plant Yielding Potential Anti-cancer Drugs Vasanthakumar K1, Sumesh Kumar S*2 and Tsegay Shimelis3 1Professor of the Horticulture Program, Haramaya University, Ethiopia 2Asst Professor in Psychiatric Nursing, Haramaya University, Ethiopia 3Graduate Assistant of the Horticulture Program, Haramaya University, Ethiopia Received: December 01, 2017; Published: December 06, 2017 *Corresponding author: S Sumesh Kumar, Asst Professor in Psychiatric Nursing , School Of Nursing and Midwifery, Haramaya University, Ethiopia, Tel : ; Email: Introduction There can be many years between promising laboratory work Maytenus ovatus (Schweinf.) of the family Celastraceous is a and the availability of an effective anti-cancer drug. In the 1950’s scientists began systematically examining natural organisms as a and is widespread in the savannah regions of tropical Africa [1]. shrub usually spiny with whitish flowers bearing reddish fruits source of useful anti-cancer substances [6]. It has recently been Mountains and sub-mountainous regions of African countries, argued that “the use of natural products has been the single most viz., Ethiopia, Kenya, Tanzania, Uganda, Mozambique and others successful strategy in the discovery of novel anti-cancer medicines”. are wild habitats for the species, Maytenus ovatus, M. serratus, M. These phyto-chemicals that is selectively more toxic to cancer cells heterophylla and M. senegalensis [2]. Maytansine, a benzo-ansa- than normal cells have been used in screening programs and are macrolide (ansamycin antibiotic) is a highly potent microtubule- developed as potential chemotherapy drugs.