Specific Receptors for the Chemokines CXCR2 and CXCR4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Article Tocilizumab Infusion Therapy Normalizes Inflammation in Sporadic ALS Patients
Am J Neurodegener Dis 2013;2(2):129-139 www.AJND.us /ISSN:2165-591X/AJND1304002 Original Article Tocilizumab infusion therapy normalizes inflammation in sporadic ALS patients Milan Fiala1, Mathew T Mizwicki1, Rachel Weitzman1, Larry Magpantay2, Norihiro Nishimoto3 1Department of Surgery, David Geffen School of Medicine at UCLA, 100 UCLA Medical Plaza, Suite 220, Los Angeles, CA 90095-6970, USA; 2Department of Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, Los Angeles, 650 Charles E. Young Drive, Los Angeles, CA, 90095-1735, USA; 3Department of Molecular Regulation for Intractable Diseases, Institute of Medical Sciences, Tokyo Medical University, Minamisenba, Chuo- ku, Osaka, 542-0081, Japan Received April 8 2013; Accepted May 19 2013; Epub June 21, 2013; Published July 1, 2013 Abstract: Patients with sporadic amyotrophic lateral sclerosis (sALS) show inflammation in the spinal cord and pe- ripheral blood. The inflammation is driven by stimulation of macrophages by aggregated superoxide dismutase 1 (SOD1) through caspase1, interleukin 1 (IL1), IL6 and chemokine signaling. Inflammatory gene activation is inhibit- ed in vitro by tocilizumab, a humanized antibody to IL6 receptor (IL6R). Tocilizumab inhibits global interleukin-6 (IL6) signaling, a key mechanism in chronic rheumatoid disorders. Here we studied in vivo baseline inflammatory gene transcription in peripheral blood mononuclear cells (PBMCs) of 10 sALS patients, and the effects of tocilizumab (ActemraR) infusions. At baseline, one half of ALS subjects had strong inflammatory activation (Group 1) (8 genes up regulated >4-fold, P<0.05 vs. controls) and the other half (Group 2) had weak activation. All patients showed greater than four-fold up regulation of MMP1, CCL7, CCL13 and CCL24. -
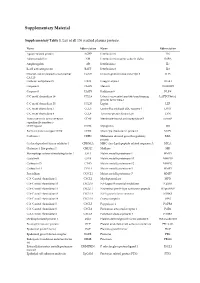
Supplementary Material
Supplementary Material Supplementary Table 1. List of all 136 studied plasma proteins. Name Abbreviation Name Abbreviation Agouti-related protein AGRP Interleukin-6 IL6 Adrenomedullin AM Interleukin-6 receptor subunit alpha IL6RA Amphiregulin AR Interleukin-7 IL7 B-cell activating factor BAFF Interleukin-8 IL8 Ovarian cancer-related tumor marker CA125 Immunoglobulin-like transcript 3 ILT3 CA 125 Carbonic anhydrase IX CAIX Integrin alpha-1 ITGA1 Caspase-3 CASP3 Melusin ITGB1BP2 Caspase-8 CASP8 Kallikrein-6 KLK6 C-C motif chemokine 19 CCL19 Latency-associated peptide transforming LAPTGFbeta1 growth factor beta-1 C-C motif chemokine 20 CCL20 Leptin LEP C-C motif chemokine 3 CCL3 Lectin-like oxidized LDL receptor 1 LOX1 C-C motif chemokine 4 CCL4 Tyrosine-protein kinase Lyn LYN Tumor necrosis factor receptor CD40 Membrane-bound aminopeptidase P mAmP superfamily member 5 CD40 ligand CD40L Myoglobin MB Early activation antigen CD69 CD69 Monocyte chemotactic protein 1 MCP1 Cadherin-3 CDH3 Melanoma-derived growth regulatory MIA protein Cyclin-dependent kinase inhibitor 1 CDKN1A MHC class I polypeptide-related sequence A MICA Chitinase-3-like protein 1 CHI3L1 Midkine MK Macrophage colony-stimulating factor 1 CSF1 Matrix metalloproteinase-1 MMP1 Cystatin-B CSTB Matrix metalloproteinase-10 MMP10 Cathepsin D CTSD Matrix metalloproteinase-12 MMP12 Cathepsin L1 CTSL1 Matrix metalloproteinase-3 MMP3 Fractalkine CX3CL1 Matrix metalloproteinase-7 MMP7 C-X-C motif chemokine 1 CXCL1 Myeloperoxidase MPO C-X-C motif chemokine 10 CXCL10 NF-kappa-B essential -

The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature
International Journal of Molecular Sciences Review The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature Jan Korbecki 1 , Klaudyna Kojder 2, Patrycja Kapczuk 1, Patrycja Kupnicka 1 , Barbara Gawro ´nska-Szklarz 3 , Izabela Gutowska 4 , Dariusz Chlubek 1 and Irena Baranowska-Bosiacka 1,* 1 Department of Biochemistry and Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] (J.K.); [email protected] (P.K.); [email protected] (P.K.); [email protected] (D.C.) 2 Department of Anaesthesiology and Intensive Care, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71-281 Szczecin, Poland; [email protected] 3 Department of Pharmacokinetics and Therapeutic Drug Monitoring, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] 4 Department of Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wlkp. 72 Av., 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-914661515 Abstract: Hypoxia is an integral component of the tumor microenvironment. Either as chronic or cycling hypoxia, it exerts a similar effect on cancer processes by activating hypoxia-inducible factor-1 (HIF-1) and nuclear factor (NF-κB), with cycling hypoxia showing a stronger proinflammatory influ- ence. One of the systems affected by hypoxia is the CXC chemokine system. This paper reviews all available information on hypoxia-induced changes in the expression of all CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8 (IL-8), CXCL9, CXCL10, CXCL11, CXCL12 Citation: Korbecki, J.; Kojder, K.; Kapczuk, P.; Kupnicka, P.; (SDF-1), CXCL13, CXCL14, CXCL15, CXCL16, CXCL17) as well as CXC chemokine receptors— Gawro´nska-Szklarz,B.; Gutowska, I.; CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CXCR7 and CXCR8. -

Exploration of Prognostic Biomarkers and Therapeutic Targets in the Microenvironment of Bladder Cancer Based on CXC Chemokines
Exploration of Prognostic Biomarkers and Therapeutic Targets in The Microenvironment of Bladder Cancer Based on CXC Chemokines Xiaoqi Sun Department of Urology, Kaiping Central Hospital, Kaiping, 529300, China Qunxi Chen Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Lihong Zhang Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Jiewei Chen Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Xinke Zhang ( [email protected] ) Sun Yat-sen University Cancer Center Research Keywords: Bladder cancer, Biomarkers, CXC Chemokines, Microenvironment Posted Date: February 24th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-223127/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/29 Abstract Background: Bladder cancer (BLCA) has a high rate of morbidity and mortality, and is considered as one of the most malignant tumors of the urinary system. Tumor cells interact with surrounding interstitial cells, playing a key role in carcinogenesis and progression, which is partly mediated by chemokines. CXC chemokines exert anti‐tumor biological roles in the tumor microenvironment and affect patient prognosis. Nevertheless, their expression and prognostic values patients with BLCA remain unclear. Methods: We used online tools, including Oncomine, UALCAN, GEPIA, GEO databases, cBioPortal, GeneMANIA, DAVID 6.8, Metascape, TRUST (version 2.0), LinkedOmics, TCGA, and TIMER2.0 to perform the relevant analysis. Results: The mRNA levels of C-X-C motif chemokine ligand (CXCL)1, CXCL5, CXCL6, CXCL7, CXCL9, CXCL10, CXCL11, CXCL13, CXCL16, and CXCL17 were increased signicantly increased, and those of CXCL2, CXCL3, and CXCL12 were decreased signicantly in BLCA tissues as assessed using the Oncomine, TCGA, and GEO databases. -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Bio-Plex Pro™ Human Chemokine Panel
Acute Phase Response Cancer Cardiovascular Disease Cytokines, Chemokines, Growth Factors Diabetes Toxicology ™ Genotyping Bio-Plex Pro Immunoglobulin Isotyping Signal Transduction Human Chemokine Panel 6Ckine / CCL21, BCA-1 / CXCL13, CTACK / CCL27, ENA-78 / CXCL5, Eotaxin / CCL11, Eotaxin-2 / CCL24, Eotaxin-3 / CCL26, Fractalkine / CX3CL1, GCP-2 / CXCL6, GM-CSF, Gro-a / CXCL1, Gro-b / CXCL2, I-309 / CCL1, MAGNETIC SEPARATION ENABLED IFN-g, IL-1b, IL-2, IL-4, IL-6, IL-8 / CXCL8, IL-10, IL-16, IP-10 / CXCL10, I-TAC / CXCL11, MCP-1 / CCL2, MCP-2 / CCL8, MCP-3 / CCL7, MCP-4 / CCL13, MDC / CCL22, MIF, MIG / CXCL9, MIP-1a / CCL3, MIP-1d / CCL15, MIP-3a / CCL20, MIP-3b / CCL19, MPIF-1 / CCL23, SCYB16 / CXCL16, SDF-1a+b / CXCL12, TARC / CCL17, TECK / CCL25, TNF-a ■ All-in-one 40-plex kit High-Performance Multiplex Rigorous Assay Validation ■ Custom Immunoassays for Research All Bio-Plex Pro Assays undergo rigorous configurations The Bio-Plex Pro Human Chemokine evaluation that includes the following ■ Single level quality Panel features 40 magnetic bead–based parameters: control immunoassays to measure chemokines and ■■ Specificity (cross-reactivity) ■ Magnetic workflow chemotaxis-relevant analytes. The assays ■■ Accuracy (recovery) in key sample matrices have undergone rigorous evaluation to provide ■■ Inter- and intra-assay precision reliable performance for your research needs ■■ Sensitivity (limit of detection, LOD) and are available in flexible, customized formats. ■■ Assay working range (LLOQ/ULOQ) Chemokines are typically associated with the ■■ Linearity of dilution following areas of research: ■■ Parallelism and matrix effect ■■ Cancer/angiogenesis ■■ Performance characteristics in real samples ■■ Cardiovascular disease Assay Performance Definitions ■■ Autoimmune disorders The following parameters are indicative of ■■ Allergy/asthma assay performance, as shown in Table 1. -

Cytokines Explored in Saliva and Tears from Radiated Cancer Patients Correlate with Clinical Manifestations, Influencing Importa
cells Article Cytokines Explored in Saliva and Tears from Radiated Cancer Patients Correlate with Clinical Manifestations, Influencing Important Immunoregulatory Cellular Pathways Lara A. Aqrawi 1,2 , Xiangjun Chen 1, Håvard Hynne 1, Cecilie Amdal 3, Sjur Reppe 4 , Hans Christian D. Aass 4, Morten Rykke 5, Lene Hystad Hove 5, Alix Young 5, Bente Brokstad Herlofson 1,6, Kristine Løken Westgaard 1,6, Tor Paaske Utheim 4,7,8,9, Hilde Kanli Galtung 8,* and Janicke Liaaen Jensen 1 1 Department of Oral Surgery and Oral Medicine, Faculty of Dentistry, University of Oslo, 0317 Oslo, Norway; [email protected] (L.A.A.); [email protected] (X.C.); [email protected] (H.H.); [email protected] (B.B.H.); [email protected] (K.L.W.); [email protected] (J.L.J.) 2 Department of Health Sciences, Kristiania University College, 0153 Oslo, Norway 3 Section for Head and Neck Oncology, Oslo University Hospital, 0379 Oslo, Norway; [email protected] 4 Department of Medical Biochemistry, Oslo University Hospital, 0450 Oslo, Norway; [email protected] (S.R.); [email protected] (H.C.D.A.); [email protected] (T.P.U.) 5 Department of Cariology and Gerodontology, Faculty of Dentistry, University of Oslo, 0455 Oslo, Norway; [email protected] (M.R.); [email protected] (L.H.H.); [email protected] (A.Y.) 6 Department of Otorhinolaryngology-Head and Neck Surgery Division for Head, Neck and Reconstructive Surgery, Oslo University Hospital, 0450 Oslo, Norway 7 Department of Plastic and Reconstructive -

And Nuclear Factor-Κb–Regulated CXC Chemokine Gene Expression in Lung Carcinogenesis
Cancer Prevention Research Cyclic AMP-Responsive Element Binding Protein– and Nuclear Factor-κB–Regulated CXC Chemokine Gene Expression in Lung Carcinogenesis Hongxia Sun, Wen-Cheng Chung, Seung-Hee Ryu, Zhenlin Ju, Hai T. Tran, Edward Kim, Jonathan M. Kurie and Ja Seok Koo Abstract The recognition of the importance of angiogenesis in tumor progression has led to the development of antiangiogenesis as a new strategy for cancer treatment and prevention. By modulating tumor microenvironment and inducing angiogenesis, the proinflammatory cytokine interleukine (IL)-1β has been reported to promote tumor development. However, the factors mediating IL-1β–induced angiogenesis in non–small cell lung cancer (NSCLC) and the regulation of these angiogenicfactorsby IL-1 β are less clear. Here, we report that IL-1β up-regulated an array of proangiogenic CXC chemokine genes in the NSCLC cell line A549 and in normal human tracheobronchial epithelium cells, as determined by microarray analysis. Further analysis revealed that IL-1β induced much higher protein levels of CXC chemokines in NSCLC cells than in normal human tracheobronchial epithelium cells. Con- ditioned medium from IL-1β–treated A549 cells markedly increased endothelial cell migra- tion, which was suppressed by neutralizing antibodies against CXCL5 and CXCR2. We also found that IL-1β–induced CXC chemokine gene overexpression in NSCLC cells was abro- gated with the knockdown of cyclic AMP-responsive element binding protein (CREB) or nuclear factor κB (NF-κB). Moreover, the expression of the CXC chemokine genes as well as CREB and NF-κB activities was greatly increased in the tumorigenic NSCLC cell line compared with normal, premalignant immortalized or nontumorigenic cell lines. -

Receptor CCR3 on Airway Epithelial Cells Functional Analysis of The
Functional Analysis of the Chemokine Receptor CCR3 on Airway Epithelial Cells Lisa A. Beck, Brian Tancowny, Mary E. Brummet, S. Yukiko Asaki, Stephanie L. Curry, Margaret B. Penno, This information is current as Martyn Foster, Ash Bahl and Cristiana Stellato of September 29, 2021. J Immunol 2006; 177:3344-3354; ; doi: 10.4049/jimmunol.177.5.3344 http://www.jimmunol.org/content/177/5/3344 Downloaded from References This article cites 55 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/177/5/3344.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 29, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Functional Analysis of the Chemokine Receptor CCR3 on Airway Epithelial Cells Lisa A. Beck,*† Brian Tancowny,* Mary E. Brummet,* S. Yukiko Asaki,* Stephanie L. Curry,* Margaret B. Penno,‡ Martyn Foster,§ Ash Bahl,§ and Cristiana Stellato1,2* The function of chemokine receptors on structural cells is only partially known. -

CXCL8) Signaling in Cancer
Pharmaceuticals 2013, 6, 929-959; doi:10.3390/ph6080929 OPEN ACCESS pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review Rationale and Means to Target Pro-Inflammatory Interleukin-8 (CXCL8) Signaling in Cancer Laura M. Campbell, Pamela J. Maxwell and David J.J. Waugh * Centre for Cancer Research and Cell Biology, Queen’s University Belfast, 97 Lisburn Road, Belfast, Northern Ireland, BT9 7BL, UK; E-Mails: [email protected] (L.M.C.); [email protected] (P.J.M.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +44-(0)2890-972-763; Fax: +44-(0)2890-972-776. Received: 9 May 2013; in revised form: 3 July 2013 / Accepted: 29 July 2013 / Published: 6 August 2013 Abstract: It is well established that chronic inflammation underpins the development of a number of human cancers, with pro-inflammatory signaling within the tumor microenvironment contributing to tumor progression and metastasis. CXCL8 is an ELR+ pro-inflammatory CXC-chemokine which mediates its effects via signaling through two G protein-coupled receptors, CXCR1 and CXCR2. Elevated CXCL8-CXCR1/2 signaling within the tumor microenvironment of numerous cancers is known to enhance tumor progression via activation of signaling pathways promoting proliferation, angiogenesis, migration, invasion and cell survival. This review provides an overview of established roles of CXCL8-CXCR1/2 signaling in cancer and subsequently, discusses the possible strategies of targeting CXCL8-CXCR1/2 signaling in cancer, covering indirect strategies (e.g., anti-inflammatories, NFκB inhibitors) and direct CXCL8 or CXCR1/2 inhibition (e.g., neutralizing antibodies, small molecule receptor antagonists, pepducin inhibitors and siRNA strategies). -

AXIN1 in Plasma Or Serum Is a Potential New Biomarker for Endometriosis
Supplementary Materials AXIN1 in Plasma or Serum Is a Potential New Biomarker for Endometriosis Table S1. Inflammation-related proteins measured by Proximity Extension Assay. Protein Abbreviation Interleukin-8 IL-8 Vascular endothelial growth factor A VEGF-A Brain-derived neurotrophic factor BDNF Monocyte chemotactic protein 3 MCP-3 Glial cell line-derived neurotrophic factor hGDNF CUB domain-containing protein 1 CDCP1 Natural killer cell receptor 2B4 CD244 Interleukin-7 IL-7 Osteoprotegerin OPG Latency-associated peptide transforming growth factor beta 1 LAP TGF-beta-1 Urokinase-type plasminogen activator uPA Interleukin-6 IL-6 Interleukin-17C IL-17C Monocyte chemotactic protein 1 MCP-1 Interleukin-17A IL-17A C-X-C motif chemokine 11 CXCL11 Axin-1 AXIN1 TNF-related apoptosis-inducing ligand TRAIL Interleukin-20 receptor subunit alpha IL-20RA C-X-C motif chemokine 9 CXCL9 Cystatin D CST5 Interleukin-2 receptor subunit beta IL-2RB Interleukin-1 alpha IL-1 alpha Oncostatin-M OSM Interleukin-2 IL-2 C-X-C motif chemokine 1 CXCL1 Thymic stromal lymphopoietin TSLP C-C motif chemokine 4 CCL4 T cell surface glycoprotein CD6 isoform CD6 Stem cell factor SCF Interleukin-18 IL-18 Signaling lymphocytic activation molecule SLAMF1 Transforming growth factor alpha TGF-alpha Monocyte chemotactic protein 4 MCP-4 Eotaxin-1 CCL11 Tumor necrosis factor ligand superfamily member 14 TNFSF14 Fibroblast growth factor 23 FGF-23 Interleukin-10 receptor subunit alpha IL-10RA Fibroblast growth factor 5 FGF-5 Matrix metalloproteinase-1 MMP-1 Leukemia inhibitory factor -

A Homeostatic Function of CXCR2 Signalling in Articular Cartilage
Basic and translational research Ann Rheum Dis: first published as 10.1136/annrheumdis-2014-205546 on 18 August 2014. Downloaded from EXTENDED REPORT A homeostatic function of CXCR2 signalling in articular cartilage Joanna Sherwood,1,2 Jessica Bertrand,2 Giovanna Nalesso,1 Blandine Poulet,3 Andrew Pitsillides,4 Laura Brandolini,5 Alexandra Karystinou,6 Cosimo De Bari,6 Frank P Luyten,7 Costantino Pitzalis,1 Thomas Pap,2 Francesco Dell’Accio1 Handling editor Tore K Kvien ABSTRACT Metalloproteinase and aggrecanase-mediated ▸ Additional material is Objective ELR+ CXC chemokines are heparin-binding extracellular matrix (ECM) degradation, and chon- published online only. To view cytokines signalling through the CXCR1 and CXCR2 drocyte apoptosis all contribute to cartilage break- please visit the journal online receptors. ELR+ CXC chemokines have been associated down and are initially compensated by chondrocyte (http://dx.doi.org/10.1136/ with inflammatory arthritis due to their capacity to proliferation and upregulation of SOX9, which dir- annrheumdis-2014-205546). attract inflammatory cells. Here, we describe an ectly regulates the synthesis of major ECM compo- fi – For numbered af liations see unsuspected physiological function of these molecules in nents including aggrecan and type II collagen.2 4 end of article. articular cartilage homeostasis. When such compensatory mechanisms are impaired Correspondence to Methods Chemokine receptors and ligands were or insufficient, cartilage breakdown progresses and Professor Francesco Dell’Accio, detected by immunohistochemistry, western blotting and ultimately leads to joint failure. Supporting the Centre for Experimental RT-PCR. Osteoarthritis was induced in wild-type and homeostatic response of cartilage can slow down or − − Medicine and Rheumatology, CXCR2 / mice by destabilisation of the medial even revert cartilage degeneration in animal William Harvey Research 56 Institute, Barts and the London meniscus (DMM).