The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prognostic Value of CXCL17 and CXCR8 Expression in Patients with Colon Cancer
ONCOLOGY LETTERS 20: 2711-2720, 2020 Prognostic value of CXCL17 and CXCR8 expression in patients with colon cancer HONGYAN YAO1,2, YUFENG LV3, XUEFENG BAI4, ZHAOJIN YU4 and XIAOJIAN LIU1 1Department of Pharmacology, School of Basic Medical Sciences, Jinzhou Medical University; 2Nuclear Medicine Department, Jinzhou Central Hospital, Jinzhou, Liaoning 121001; 3Department of Respiration and Critical Care, The Affiliated Hongqi Hospital of Mudanjiang Medical University, Mudanjiang, Heilongjiang 157011; 4Department of Pharmacology, School of Pharmacy, China Medical University, Shenyang, Liaoning 110001, P.R. China Received October 11, 2019; Accepted May 27, 2020 DOI: 10.3892/ol.2020.11819 Abstract. C-X-C motif chemokine ligand 17 (CXCL17) is a CXCL17/CXCR8 signalling may be involved in colon cancer mucous chemokine and its expression is highly correlated with and contribute to improved patient outcomes. that of G protein-coupled receptor 35 (GPR35), which has been confirmed as its receptor and named C‑X‑C motif chemokine Introduction receptor 8 (CXCR8). CXCL17 is upregulated in several types of cancer. However, the biological role of this chemokine Chemokines are a large family of cytokines serving important in colon cancer remains unknown. In the present study, the roles in the immune system, such as the regulation of cell traf- expression levels of CXCL17 and CXCR8 were examined ficking to inflammatory sites (1). By regulating the activity of using immunohistochemistry in 101 colon cancer tissues and cells in homeostasis and signal transduction, chemokines are 79 healthy tumour-adjacent tissues. CXCL17 and CXCR8 involved in numerous physiological and pathological processes expression levels were increased in the colon cancer samples such as angiogenesis, embryonic development, wound healing, compared with tumour-adjacent samples. -

Induces Homing of Antigen-Specific and Non Chemokine-Adjuvanted
Chemokine-Adjuvanted Plasmid DNA Induces Homing of Antigen-Specific and Non −Antigen-Specific B and T Cells to the Intestinal and Genital Mucosae This information is current as of September 25, 2021. Yoann Aldon, Sven Kratochvil, Robin J. Shattock and Paul F. McKay J Immunol published online 8 January 2020 http://www.jimmunol.org/content/early/2020/01/07/jimmun ol.1901184 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2020/01/07/jimmunol.190118 Material 4.DCSupplemental http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 25, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Author Choice Freely available online through The Journal of Immunology Author Choice option Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2020 The Authors All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published January 8, 2020, doi:10.4049/jimmunol.1901184 The Journal of Immunology Chemokine-Adjuvanted Plasmid DNA Induces Homing of Antigen-Specific and Non–Antigen-Specific B and T Cells to the Intestinal and Genital Mucosae Yoann Aldon, Sven Kratochvil, Robin J. -

In a Lung Disease Model an Anti-Inflammatory Activity Of
Mouse ChemR23 Is Expressed in Dendritic Cell Subsets and Macrophages, and Mediates an Anti-Inflammatory Activity of Chemerin in a Lung Disease Model This information is current as of September 29, 2021. Souphalone Luangsay, Valérie Wittamer, Benjamin Bondue, Olivier De Henau, Laurie Rouger, Maryse Brait, Jean-Denis Franssen, Patricia de Nadai, François Huaux and Marc Parmentier J Immunol 2009; 183:6489-6499; Prepublished online 19 Downloaded from October 2009; doi: 10.4049/jimmunol.0901037 http://www.jimmunol.org/content/183/10/6489 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2009/10/20/jimmunol.090103 Material 7.DC1 References This article cites 60 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/183/10/6489.full#ref-list-1 by guest on September 29, 2021 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright -

CXCL13/CXCR5 Interaction Facilitates VCAM-1-Dependent Migration in Human Osteosarcoma
International Journal of Molecular Sciences Article CXCL13/CXCR5 Interaction Facilitates VCAM-1-Dependent Migration in Human Osteosarcoma 1, 2,3,4, 5 6 7 Ju-Fang Liu y, Chiang-Wen Lee y, Chih-Yang Lin , Chia-Chia Chao , Tsung-Ming Chang , Chien-Kuo Han 8, Yuan-Li Huang 8, Yi-Chin Fong 9,10,* and Chih-Hsin Tang 8,11,12,* 1 School of Oral Hygiene, College of Oral Medicine, Taipei Medical University, Taipei City 11031, Taiwan; [email protected] 2 Department of Orthopaedic Surgery, Chang Gung Memorial Hospital, Puzi City, Chiayi County 61363, Taiwan; [email protected] 3 Department of Nursing, Division of Basic Medical Sciences, and Chronic Diseases and Health Promotion Research Center, Chang Gung University of Science and Technology, Puzi City, Chiayi County 61363, Taiwan 4 Research Center for Industry of Human Ecology and Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Guishan Dist., Taoyuan City 33303, Taiwan 5 School of Medicine, China Medical University, Taichung 40402, Taiwan; [email protected] 6 Department of Respiratory Therapy, Fu Jen Catholic University, New Taipei City 24205, Taiwan; [email protected] 7 School of Medicine, Institute of Physiology, National Yang-Ming University, Taipei City 11221, Taiwan; [email protected] 8 Department of Biotechnology, College of Health Science, Asia University, Taichung 40402, Taiwan; [email protected] (C.-K.H.); [email protected] (Y.-L.H.) 9 Department of Sports Medicine, College of Health Care, China Medical University, Taichung 40402, Taiwan 10 Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin 65152, Taiwan 11 Department of Pharmacology, School of Medicine, China Medical University, Taichung 40402, Taiwan 12 Chinese Medicine Research Center, China Medical University, Taichung 40402, Taiwan * Correspondence: [email protected] (Y.-C.F.); [email protected] (C.-H.T.); Tel.: +886-4-2205-2121-7726 (C.-H.T.); Fax: +886-4-2233-3641 (C.-H.T.) These authors contributed equally to this work. -

Cxcl17 and Its Association with T Cells
Hernández-Ruiz et al., Arch Autoimmune Archives of Autoimmune Diseases Dis 2020; 1(1):28-31. Commentary Cxcl17 and its association with T cells Marcela Hernández-Ruiz1, Albert Zlotnik2* 1Lymphotrek, San Diego, CA, United Keywords: CXCL17, Auto immunities, T cells, IL-23 States Abbreviations: BALF: Bronchoalveolar Lavage Fluid; CLP: Common Lymphoid Progenitor; CNS: 2Department of Physiology Central Nervous System; DP: Double Positive; EAE: Experimental Autoimmune Encephalomyelitis; LN: and Biophysics and Institute for Lymph Nodes; LTHSC: Long-term Hematopoietic Stem Cells; MHC: Major Histocompatibility Complex; Immunology, University of California Irvine, Irvine, CA, United States MOG: Myelin Oligodendrocyte Glycoprotein; STHCS: Short-Term Hematopoietic Stem Cells; TCR: T cell Receptor *Author for correspondence: Email: [email protected] We recently published an article describing the importance of CXCL17 in T cell responses [1]. In summary, we observed the following: Received date: September 02, 2020 Accepted date: September 23, 2020 1) Cxcl17 is necessary to maintain normal ratios of T cell subpopulations in lymph nodes (LN). 2) Cxcl17-/- mice develop more intense inflammatory responses than wild type mice. Copyright: © 2020 Hernández-Ruiz Cxcl17 is a mucosal chemokine predominantly expressed in mucosal tissues of the respiratory M, et al. This is an open-access article tract and digestive system [2-4]. However, it is also expressed in primary immune tissues such as distributed under the terms of the bone marrow and thymus (where its function is currently unknown- Table 1). Microarray data Creative Commons Attribution License, from the Immgen database of 298 purified immune cell subpopulations, and consistent with the which permits unrestricted use, distribution, and reproduction in any analysis shown in table 1, indicates that Cxcl17 is expressed in the thymus. -

Human B Cells Expression in Terminally Differentiating Induces
1,25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells This information is current as Aiko-Konno Shirakawa, Daisuke Nagakubo, Kunio of September 28, 2021. Hieshima, Takashi Nakayama, Zhe Jin and Osamu Yoshie J Immunol 2008; 180:2786-2795; ; doi: 10.4049/jimmunol.180.5.2786 http://www.jimmunol.org/content/180/5/2786 Downloaded from References This article cites 55 articles, 29 of which you can access for free at: http://www.jimmunol.org/content/180/5/2786.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology 1,25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells1 Aiko-Konno Shirakawa,2 Daisuke Nagakubo,2 Kunio Hieshima, Takashi Nakayama, Zhe Jin, and Osamu Yoshie3 In the B cell lineage, CCR10 is known to be selectively expressed by plasma cells, especially those secreting IgA. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Antimicrobial Activity As a Chemokine with Broad-Spectrum CCL28 Has
CCL28 Has Dual Roles in Mucosal Immunity as a Chemokine with Broad-Spectrum Antimicrobial Activity This information is current as Kunio Hieshima, Haruo Ohtani, Michiko Shibano, Dai of September 26, 2021. Izawa, Takashi Nakayama, Yuri Kawasaki, Fumio Shiba, Mitsuru Shiota, Fuminori Katou, Takuya Saito and Osamu Yoshie J Immunol 2003; 170:1452-1461; ; doi: 10.4049/jimmunol.170.3.1452 Downloaded from http://www.jimmunol.org/content/170/3/1452 References This article cites 36 articles, 19 of which you can access for free at: http://www.jimmunol.org/content/170/3/1452.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 26, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2003 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology CCL28 Has Dual Roles in Mucosal Immunity as a Chemokine with Broad-Spectrum Antimicrobial Activity1 Kunio Hieshima,* Haruo Ohtani,2‡ Michiko Shibano,* Dai Izawa,* Takashi Nakayama,* Yuri Kawasaki,* Fumio Shiba,* Mitsuru Shiota,† Fuminori Katou,§ Takuya Saito,¶ and Osamu Yoshie3* CCL28 is a CC chemokine signaling via CCR10 and CCR3 that is selectively expressed in certain mucosal tissues such as exocrine glands, trachea, and colon. -

Neutrophil Chemoattractant Receptors in Health and Disease: Double-Edged Swords
Cellular & Molecular Immunology www.nature.com/cmi REVIEW ARTICLE Neutrophil chemoattractant receptors in health and disease: double-edged swords Mieke Metzemaekers1, Mieke Gouwy1 and Paul Proost 1 Neutrophils are frontline cells of the innate immune system. These effector leukocytes are equipped with intriguing antimicrobial machinery and consequently display high cytotoxic potential. Accurate neutrophil recruitment is essential to combat microbes and to restore homeostasis, for inflammation modulation and resolution, wound healing and tissue repair. After fulfilling the appropriate effector functions, however, dampening neutrophil activation and infiltration is crucial to prevent damage to the host. In humans, chemoattractant molecules can be categorized into four biochemical families, i.e., chemotactic lipids, formyl peptides, complement anaphylatoxins and chemokines. They are critically involved in the tight regulation of neutrophil bone marrow storage and egress and in spatial and temporal neutrophil trafficking between organs. Chemoattractants function by activating dedicated heptahelical G protein-coupled receptors (GPCRs). In addition, emerging evidence suggests an important role for atypical chemoattractant receptors (ACKRs) that do not couple to G proteins in fine-tuning neutrophil migratory and functional responses. The expression levels of chemoattractant receptors are dependent on the level of neutrophil maturation and state of activation, with a pivotal modulatory role for the (inflammatory) environment. Here, we provide an overview -

Original Article Tocilizumab Infusion Therapy Normalizes Inflammation in Sporadic ALS Patients
Am J Neurodegener Dis 2013;2(2):129-139 www.AJND.us /ISSN:2165-591X/AJND1304002 Original Article Tocilizumab infusion therapy normalizes inflammation in sporadic ALS patients Milan Fiala1, Mathew T Mizwicki1, Rachel Weitzman1, Larry Magpantay2, Norihiro Nishimoto3 1Department of Surgery, David Geffen School of Medicine at UCLA, 100 UCLA Medical Plaza, Suite 220, Los Angeles, CA 90095-6970, USA; 2Department of Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, Los Angeles, 650 Charles E. Young Drive, Los Angeles, CA, 90095-1735, USA; 3Department of Molecular Regulation for Intractable Diseases, Institute of Medical Sciences, Tokyo Medical University, Minamisenba, Chuo- ku, Osaka, 542-0081, Japan Received April 8 2013; Accepted May 19 2013; Epub June 21, 2013; Published July 1, 2013 Abstract: Patients with sporadic amyotrophic lateral sclerosis (sALS) show inflammation in the spinal cord and pe- ripheral blood. The inflammation is driven by stimulation of macrophages by aggregated superoxide dismutase 1 (SOD1) through caspase1, interleukin 1 (IL1), IL6 and chemokine signaling. Inflammatory gene activation is inhibit- ed in vitro by tocilizumab, a humanized antibody to IL6 receptor (IL6R). Tocilizumab inhibits global interleukin-6 (IL6) signaling, a key mechanism in chronic rheumatoid disorders. Here we studied in vivo baseline inflammatory gene transcription in peripheral blood mononuclear cells (PBMCs) of 10 sALS patients, and the effects of tocilizumab (ActemraR) infusions. At baseline, one half of ALS subjects had strong inflammatory activation (Group 1) (8 genes up regulated >4-fold, P<0.05 vs. controls) and the other half (Group 2) had weak activation. All patients showed greater than four-fold up regulation of MMP1, CCL7, CCL13 and CCL24. -
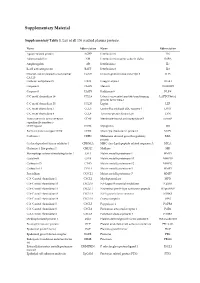
Supplementary Material
Supplementary Material Supplementary Table 1. List of all 136 studied plasma proteins. Name Abbreviation Name Abbreviation Agouti-related protein AGRP Interleukin-6 IL6 Adrenomedullin AM Interleukin-6 receptor subunit alpha IL6RA Amphiregulin AR Interleukin-7 IL7 B-cell activating factor BAFF Interleukin-8 IL8 Ovarian cancer-related tumor marker CA125 Immunoglobulin-like transcript 3 ILT3 CA 125 Carbonic anhydrase IX CAIX Integrin alpha-1 ITGA1 Caspase-3 CASP3 Melusin ITGB1BP2 Caspase-8 CASP8 Kallikrein-6 KLK6 C-C motif chemokine 19 CCL19 Latency-associated peptide transforming LAPTGFbeta1 growth factor beta-1 C-C motif chemokine 20 CCL20 Leptin LEP C-C motif chemokine 3 CCL3 Lectin-like oxidized LDL receptor 1 LOX1 C-C motif chemokine 4 CCL4 Tyrosine-protein kinase Lyn LYN Tumor necrosis factor receptor CD40 Membrane-bound aminopeptidase P mAmP superfamily member 5 CD40 ligand CD40L Myoglobin MB Early activation antigen CD69 CD69 Monocyte chemotactic protein 1 MCP1 Cadherin-3 CDH3 Melanoma-derived growth regulatory MIA protein Cyclin-dependent kinase inhibitor 1 CDKN1A MHC class I polypeptide-related sequence A MICA Chitinase-3-like protein 1 CHI3L1 Midkine MK Macrophage colony-stimulating factor 1 CSF1 Matrix metalloproteinase-1 MMP1 Cystatin-B CSTB Matrix metalloproteinase-10 MMP10 Cathepsin D CTSD Matrix metalloproteinase-12 MMP12 Cathepsin L1 CTSL1 Matrix metalloproteinase-3 MMP3 Fractalkine CX3CL1 Matrix metalloproteinase-7 MMP7 C-X-C motif chemokine 1 CXCL1 Myeloperoxidase MPO C-X-C motif chemokine 10 CXCL10 NF-kappa-B essential -

The CXCL7/CXCR1/2 Axis Is a Key Driver in the Growth of Clear Cell Renal Cell Carcinoma
Published OnlineFirst December 12, 2013; DOI: 10.1158/0008-5472.CAN-13-1267 Cancer Therapeutics, Targets, and Chemical Biology Research The CXCL7/CXCR1/2 Axis Is a Key Driver in the Growth of Clear Cell Renal Cell Carcinoma Renaud Grepin 5,Melanie Guyot1, Sandy Giuliano1, Marina Boncompagni1, Damien Ambrosetti1,2, Emmanuel Chamorey3, Jean-Yves Scoazec4, Sylvie Negrier4,Hel ene Simonnet4, and Gilles Pages 1 Abstract Mutations in the von Hippel–Lindau gene upregulate expression of the central angiogenic factor VEGF, which drives abnormal angiogenesis in clear cell renal cell carcinomas (ccRCC). However, the overexpression of VEGF in these tumors was not found to correlate with overall survival. Here, we show that the proangiogenic, proin- flammatory cytokine CXCL7 is an independent prognostic factor for overall survival in this setting. CXCL7 antibodies strongly reduced the growth of ccRCC tumors in nude mice. Conversely, conditional overexpression of CXCL7 accelerated ccRCC development. CXCL7 promoted cell proliferation in vivo and in vitro, in which expression of CXCL7 was induced by the central proinflammatory cytokine interleukin (IL)-1b. ccRCC cells normally secrete low amounts of CXCL7; it was more highly expressed in tumors due to high levels of IL-1b there. We found that a pharmacological inhibitor of the CXCL7 receptors CXCR1 and CXCR2 (SB225002) was sufficient to inhibit endothelial cell proliferation and ccRCC growth. Because CXCR1 and CXCR2 are present on both endothelial and ccRCC cells, their inhibition affected both the tumor vasculature and the proliferation of tumor cells. Our results highlight the CXCL7/CXCR1/CXCR2 axis as a pertinent target for the treatment of ccRCC.