Receptor CCR3 on Airway Epithelial Cells Functional Analysis of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutrophil Chemoattractant Receptors in Health and Disease: Double-Edged Swords
Cellular & Molecular Immunology www.nature.com/cmi REVIEW ARTICLE Neutrophil chemoattractant receptors in health and disease: double-edged swords Mieke Metzemaekers1, Mieke Gouwy1 and Paul Proost 1 Neutrophils are frontline cells of the innate immune system. These effector leukocytes are equipped with intriguing antimicrobial machinery and consequently display high cytotoxic potential. Accurate neutrophil recruitment is essential to combat microbes and to restore homeostasis, for inflammation modulation and resolution, wound healing and tissue repair. After fulfilling the appropriate effector functions, however, dampening neutrophil activation and infiltration is crucial to prevent damage to the host. In humans, chemoattractant molecules can be categorized into four biochemical families, i.e., chemotactic lipids, formyl peptides, complement anaphylatoxins and chemokines. They are critically involved in the tight regulation of neutrophil bone marrow storage and egress and in spatial and temporal neutrophil trafficking between organs. Chemoattractants function by activating dedicated heptahelical G protein-coupled receptors (GPCRs). In addition, emerging evidence suggests an important role for atypical chemoattractant receptors (ACKRs) that do not couple to G proteins in fine-tuning neutrophil migratory and functional responses. The expression levels of chemoattractant receptors are dependent on the level of neutrophil maturation and state of activation, with a pivotal modulatory role for the (inflammatory) environment. Here, we provide an overview -

Original Article Tocilizumab Infusion Therapy Normalizes Inflammation in Sporadic ALS Patients
Am J Neurodegener Dis 2013;2(2):129-139 www.AJND.us /ISSN:2165-591X/AJND1304002 Original Article Tocilizumab infusion therapy normalizes inflammation in sporadic ALS patients Milan Fiala1, Mathew T Mizwicki1, Rachel Weitzman1, Larry Magpantay2, Norihiro Nishimoto3 1Department of Surgery, David Geffen School of Medicine at UCLA, 100 UCLA Medical Plaza, Suite 220, Los Angeles, CA 90095-6970, USA; 2Department of Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, Los Angeles, 650 Charles E. Young Drive, Los Angeles, CA, 90095-1735, USA; 3Department of Molecular Regulation for Intractable Diseases, Institute of Medical Sciences, Tokyo Medical University, Minamisenba, Chuo- ku, Osaka, 542-0081, Japan Received April 8 2013; Accepted May 19 2013; Epub June 21, 2013; Published July 1, 2013 Abstract: Patients with sporadic amyotrophic lateral sclerosis (sALS) show inflammation in the spinal cord and pe- ripheral blood. The inflammation is driven by stimulation of macrophages by aggregated superoxide dismutase 1 (SOD1) through caspase1, interleukin 1 (IL1), IL6 and chemokine signaling. Inflammatory gene activation is inhibit- ed in vitro by tocilizumab, a humanized antibody to IL6 receptor (IL6R). Tocilizumab inhibits global interleukin-6 (IL6) signaling, a key mechanism in chronic rheumatoid disorders. Here we studied in vivo baseline inflammatory gene transcription in peripheral blood mononuclear cells (PBMCs) of 10 sALS patients, and the effects of tocilizumab (ActemraR) infusions. At baseline, one half of ALS subjects had strong inflammatory activation (Group 1) (8 genes up regulated >4-fold, P<0.05 vs. controls) and the other half (Group 2) had weak activation. All patients showed greater than four-fold up regulation of MMP1, CCL7, CCL13 and CCL24. -
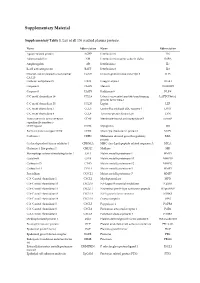
Supplementary Material
Supplementary Material Supplementary Table 1. List of all 136 studied plasma proteins. Name Abbreviation Name Abbreviation Agouti-related protein AGRP Interleukin-6 IL6 Adrenomedullin AM Interleukin-6 receptor subunit alpha IL6RA Amphiregulin AR Interleukin-7 IL7 B-cell activating factor BAFF Interleukin-8 IL8 Ovarian cancer-related tumor marker CA125 Immunoglobulin-like transcript 3 ILT3 CA 125 Carbonic anhydrase IX CAIX Integrin alpha-1 ITGA1 Caspase-3 CASP3 Melusin ITGB1BP2 Caspase-8 CASP8 Kallikrein-6 KLK6 C-C motif chemokine 19 CCL19 Latency-associated peptide transforming LAPTGFbeta1 growth factor beta-1 C-C motif chemokine 20 CCL20 Leptin LEP C-C motif chemokine 3 CCL3 Lectin-like oxidized LDL receptor 1 LOX1 C-C motif chemokine 4 CCL4 Tyrosine-protein kinase Lyn LYN Tumor necrosis factor receptor CD40 Membrane-bound aminopeptidase P mAmP superfamily member 5 CD40 ligand CD40L Myoglobin MB Early activation antigen CD69 CD69 Monocyte chemotactic protein 1 MCP1 Cadherin-3 CDH3 Melanoma-derived growth regulatory MIA protein Cyclin-dependent kinase inhibitor 1 CDKN1A MHC class I polypeptide-related sequence A MICA Chitinase-3-like protein 1 CHI3L1 Midkine MK Macrophage colony-stimulating factor 1 CSF1 Matrix metalloproteinase-1 MMP1 Cystatin-B CSTB Matrix metalloproteinase-10 MMP10 Cathepsin D CTSD Matrix metalloproteinase-12 MMP12 Cathepsin L1 CTSL1 Matrix metalloproteinase-3 MMP3 Fractalkine CX3CL1 Matrix metalloproteinase-7 MMP7 C-X-C motif chemokine 1 CXCL1 Myeloperoxidase MPO C-X-C motif chemokine 10 CXCL10 NF-kappa-B essential -

The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature
International Journal of Molecular Sciences Review The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors—A Review of Literature Jan Korbecki 1 , Klaudyna Kojder 2, Patrycja Kapczuk 1, Patrycja Kupnicka 1 , Barbara Gawro ´nska-Szklarz 3 , Izabela Gutowska 4 , Dariusz Chlubek 1 and Irena Baranowska-Bosiacka 1,* 1 Department of Biochemistry and Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] (J.K.); [email protected] (P.K.); [email protected] (P.K.); [email protected] (D.C.) 2 Department of Anaesthesiology and Intensive Care, Pomeranian Medical University in Szczecin, Unii Lubelskiej 1, 71-281 Szczecin, Poland; [email protected] 3 Department of Pharmacokinetics and Therapeutic Drug Monitoring, Pomeranian Medical University in Szczecin, Powsta´nców Wielkopolskich 72 Av., 70-111 Szczecin, Poland; [email protected] 4 Department of Medical Chemistry, Pomeranian Medical University in Szczecin, Powsta´nców Wlkp. 72 Av., 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-914661515 Abstract: Hypoxia is an integral component of the tumor microenvironment. Either as chronic or cycling hypoxia, it exerts a similar effect on cancer processes by activating hypoxia-inducible factor-1 (HIF-1) and nuclear factor (NF-κB), with cycling hypoxia showing a stronger proinflammatory influ- ence. One of the systems affected by hypoxia is the CXC chemokine system. This paper reviews all available information on hypoxia-induced changes in the expression of all CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8 (IL-8), CXCL9, CXCL10, CXCL11, CXCL12 Citation: Korbecki, J.; Kojder, K.; Kapczuk, P.; Kupnicka, P.; (SDF-1), CXCL13, CXCL14, CXCL15, CXCL16, CXCL17) as well as CXC chemokine receptors— Gawro´nska-Szklarz,B.; Gutowska, I.; CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CXCR7 and CXCR8. -

Exploration of Prognostic Biomarkers and Therapeutic Targets in the Microenvironment of Bladder Cancer Based on CXC Chemokines
Exploration of Prognostic Biomarkers and Therapeutic Targets in The Microenvironment of Bladder Cancer Based on CXC Chemokines Xiaoqi Sun Department of Urology, Kaiping Central Hospital, Kaiping, 529300, China Qunxi Chen Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Lihong Zhang Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Jiewei Chen Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China Xinke Zhang ( [email protected] ) Sun Yat-sen University Cancer Center Research Keywords: Bladder cancer, Biomarkers, CXC Chemokines, Microenvironment Posted Date: February 24th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-223127/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/29 Abstract Background: Bladder cancer (BLCA) has a high rate of morbidity and mortality, and is considered as one of the most malignant tumors of the urinary system. Tumor cells interact with surrounding interstitial cells, playing a key role in carcinogenesis and progression, which is partly mediated by chemokines. CXC chemokines exert anti‐tumor biological roles in the tumor microenvironment and affect patient prognosis. Nevertheless, their expression and prognostic values patients with BLCA remain unclear. Methods: We used online tools, including Oncomine, UALCAN, GEPIA, GEO databases, cBioPortal, GeneMANIA, DAVID 6.8, Metascape, TRUST (version 2.0), LinkedOmics, TCGA, and TIMER2.0 to perform the relevant analysis. Results: The mRNA levels of C-X-C motif chemokine ligand (CXCL)1, CXCL5, CXCL6, CXCL7, CXCL9, CXCL10, CXCL11, CXCL13, CXCL16, and CXCL17 were increased signicantly increased, and those of CXCL2, CXCL3, and CXCL12 were decreased signicantly in BLCA tissues as assessed using the Oncomine, TCGA, and GEO databases. -

Part One Fundamentals of Chemokines and Chemokine Receptors
Part One Fundamentals of Chemokines and Chemokine Receptors Chemokine Receptors as Drug Targets. Edited by Martine J. Smit, Sergio A. Lira, and Rob Leurs Copyright Ó 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 978-3-527-32118-6 j3 1 Structural Aspects of Chemokines and their Interactions with Receptors and Glycosaminoglycans Amanda E. I. Proudfoot, India Severin, Damon Hamel, and Tracy M. Handel 1.1 Introduction Chemokines are a large subfamily of cytokines (50 in humans) that can be distinguished from other cytokines due to several features. They share a common biological activity, which is the control of the directional migration of leukocytes, hence their name, chemoattractant cytokines. They are all small proteins (approx. 8 kDa) that are highly basic, with two exceptions (MIP-1a, MIP-1b). Also, they have a highly conserved monomeric fold, constrained by 1–3 disulfides which are formed from a conserved pattern of cysteine residues (the majority of chemokines have four cysteines). The pattern of cysteine residues is used as the basis of their division into subclasses and for their nomenclature. The first class, referred to as CXC or a-chemokines, have a single residue between the first N-terminal Cys residues, whereas in the CC class, or b-chemokines, these two Cys residues are adjacent. While most chemokines have two disulfides, the CC subclass also has three members that contain three. Subsequent to the CC and CXC families, two fi additional subclasses were identi ed, the CX3C subclass [1, 2], which has three amino acids separating the N-terminal Cys pair, and the C subclass, which has a single disulfide. -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Bio-Plex Pro™ Human Chemokine Panel
Acute Phase Response Cancer Cardiovascular Disease Cytokines, Chemokines, Growth Factors Diabetes Toxicology ™ Genotyping Bio-Plex Pro Immunoglobulin Isotyping Signal Transduction Human Chemokine Panel 6Ckine / CCL21, BCA-1 / CXCL13, CTACK / CCL27, ENA-78 / CXCL5, Eotaxin / CCL11, Eotaxin-2 / CCL24, Eotaxin-3 / CCL26, Fractalkine / CX3CL1, GCP-2 / CXCL6, GM-CSF, Gro-a / CXCL1, Gro-b / CXCL2, I-309 / CCL1, MAGNETIC SEPARATION ENABLED IFN-g, IL-1b, IL-2, IL-4, IL-6, IL-8 / CXCL8, IL-10, IL-16, IP-10 / CXCL10, I-TAC / CXCL11, MCP-1 / CCL2, MCP-2 / CCL8, MCP-3 / CCL7, MCP-4 / CCL13, MDC / CCL22, MIF, MIG / CXCL9, MIP-1a / CCL3, MIP-1d / CCL15, MIP-3a / CCL20, MIP-3b / CCL19, MPIF-1 / CCL23, SCYB16 / CXCL16, SDF-1a+b / CXCL12, TARC / CCL17, TECK / CCL25, TNF-a ■ All-in-one 40-plex kit High-Performance Multiplex Rigorous Assay Validation ■ Custom Immunoassays for Research All Bio-Plex Pro Assays undergo rigorous configurations The Bio-Plex Pro Human Chemokine evaluation that includes the following ■ Single level quality Panel features 40 magnetic bead–based parameters: control immunoassays to measure chemokines and ■■ Specificity (cross-reactivity) ■ Magnetic workflow chemotaxis-relevant analytes. The assays ■■ Accuracy (recovery) in key sample matrices have undergone rigorous evaluation to provide ■■ Inter- and intra-assay precision reliable performance for your research needs ■■ Sensitivity (limit of detection, LOD) and are available in flexible, customized formats. ■■ Assay working range (LLOQ/ULOQ) Chemokines are typically associated with the ■■ Linearity of dilution following areas of research: ■■ Parallelism and matrix effect ■■ Cancer/angiogenesis ■■ Performance characteristics in real samples ■■ Cardiovascular disease Assay Performance Definitions ■■ Autoimmune disorders The following parameters are indicative of ■■ Allergy/asthma assay performance, as shown in Table 1. -

Recruitment and Expansion of Tregs Cells in the Tumor Environment—How to Target Them?
cancers Review Recruitment and Expansion of Tregs Cells in the Tumor Environment—How to Target Them? Justine Cinier 1,†, Margaux Hubert 1,†, Laurie Besson 1, Anthony Di Roio 1 ,Céline Rodriguez 1, Vincent Lombardi 2, Christophe Caux 1,‡ and Christine Ménétrier-Caux 1,*,‡ 1 University of Lyon, Claude Bernard Lyon 1 University, INSERM U-1052, CNRS 5286 Centre Léon Bérard, Cancer Research Center of Lyon (CRCL), 69008 Lyon, France; [email protected] (J.C.); [email protected] (M.H.); [email protected] (L.B.); [email protected] (A.D.R.); [email protected] (C.R.); [email protected] (C.C.) 2 Institut de Recherche Servier, 125 Chemin de Ronde, 78290 Croissy-sur-Seine, France; [email protected] * Correspondence: [email protected] † Co-first authorship. ‡ Co-last authorship. Simple Summary: The immune response against cancer is generated by effector T cells, among them cytotoxic CD8+ T cells that destroy cancer cells and helper CD4+ T cells that mediate and support the immune response. This antitumor function of T cells is tightly regulated by a particular subset of CD4+ T cells, named regulatory T cells (Tregs), through different mechanisms. Even if the complete inhibition of Tregs would be extremely harmful due to their tolerogenic role in impeding autoimmune diseases in the periphery, the targeted blockade of their accumulation at tumor sites or their targeted Citation: Cinier, J.; Hubert, M.; depletion represent a major therapeutic challenge. This review focuses on the mechanisms favoring Besson, L.; Di Roio, A.; Rodriguez, C.; Treg recruitment, expansion and stabilization in the tumor microenvironment and the therapeutic Lombardi, V.; Caux, C.; strategies developed to block these mechanisms. -

Cytokines Explored in Saliva and Tears from Radiated Cancer Patients Correlate with Clinical Manifestations, Influencing Importa
cells Article Cytokines Explored in Saliva and Tears from Radiated Cancer Patients Correlate with Clinical Manifestations, Influencing Important Immunoregulatory Cellular Pathways Lara A. Aqrawi 1,2 , Xiangjun Chen 1, Håvard Hynne 1, Cecilie Amdal 3, Sjur Reppe 4 , Hans Christian D. Aass 4, Morten Rykke 5, Lene Hystad Hove 5, Alix Young 5, Bente Brokstad Herlofson 1,6, Kristine Løken Westgaard 1,6, Tor Paaske Utheim 4,7,8,9, Hilde Kanli Galtung 8,* and Janicke Liaaen Jensen 1 1 Department of Oral Surgery and Oral Medicine, Faculty of Dentistry, University of Oslo, 0317 Oslo, Norway; [email protected] (L.A.A.); [email protected] (X.C.); [email protected] (H.H.); [email protected] (B.B.H.); [email protected] (K.L.W.); [email protected] (J.L.J.) 2 Department of Health Sciences, Kristiania University College, 0153 Oslo, Norway 3 Section for Head and Neck Oncology, Oslo University Hospital, 0379 Oslo, Norway; [email protected] 4 Department of Medical Biochemistry, Oslo University Hospital, 0450 Oslo, Norway; [email protected] (S.R.); [email protected] (H.C.D.A.); [email protected] (T.P.U.) 5 Department of Cariology and Gerodontology, Faculty of Dentistry, University of Oslo, 0455 Oslo, Norway; [email protected] (M.R.); [email protected] (L.H.H.); [email protected] (A.Y.) 6 Department of Otorhinolaryngology-Head and Neck Surgery Division for Head, Neck and Reconstructive Surgery, Oslo University Hospital, 0450 Oslo, Norway 7 Department of Plastic and Reconstructive -

An Introduction to Chemokines and Their Roles in Transfusion Medicine
Vox Sanguinis (2009) 96, 183–198 © 2008 The Author(s) REVIEW Journal compilation © 2008 Blackwell Publishing Ltd. DOI: 10.1111/j.1423-0410.2008.01127.x AnBlackwell Publishing Ltd introduction to chemokines and their roles in transfusion medicine R. D. Davenport Blood Bank and Transfusion Service, University of Michigan Health System, Ann Arbor, MI, USA Chemokines are a set of structurally related peptides that were first characterized as chemoattractants and have subsequently been shown to have many functions in homeostasis and pathophysiology. Diversity and redundancy of chemokine function is imparted by both selectivity and overlap in the specificity of chemokine receptors for their ligands. Chemokines have roles impacting transfusion medicine in haemat- opoiesis, haematologic malignancies, transfusion reactions, graft-versus-host disease, and viral infections. In haematopoietic cell transplantation, chemokines are active in mobilization and homing of progenitor cells, as well as mediating T-cell recruitment in graft-versus-host disease. Platelets are rich source of chemokines that recruit and activate leucocytes during thrombosis. Important transfusion-transmissible viruses such as cytomegalovirus and human immunodeficiency virus exploit chemokine receptors to evade host immunity. Chemokines may also have roles in the pathophysiology of Received: 4 June 2007, revised 29 September 2008, haemolytic and non-haemolytic transfusion reactions. accepted 16 October 2008, Key words: Chemokines, chemokine receptors, haematopoietic stem cell transplantation, published online 8 December 2008 graft-vs-host disease, transfusion reactions. General characteristics of chemokines This classification is not completely definite, as under some conditions homeostatic chemokines are inducible. Chemokines are small, secreted proteins in the range of 8– Most chemokines were originally named for their first 10 kDa that have numerous functions in normal physiology identified biological activity, such as monocyte chemoattractant and pathology. -

And Nuclear Factor-Κb–Regulated CXC Chemokine Gene Expression in Lung Carcinogenesis
Cancer Prevention Research Cyclic AMP-Responsive Element Binding Protein– and Nuclear Factor-κB–Regulated CXC Chemokine Gene Expression in Lung Carcinogenesis Hongxia Sun, Wen-Cheng Chung, Seung-Hee Ryu, Zhenlin Ju, Hai T. Tran, Edward Kim, Jonathan M. Kurie and Ja Seok Koo Abstract The recognition of the importance of angiogenesis in tumor progression has led to the development of antiangiogenesis as a new strategy for cancer treatment and prevention. By modulating tumor microenvironment and inducing angiogenesis, the proinflammatory cytokine interleukine (IL)-1β has been reported to promote tumor development. However, the factors mediating IL-1β–induced angiogenesis in non–small cell lung cancer (NSCLC) and the regulation of these angiogenicfactorsby IL-1 β are less clear. Here, we report that IL-1β up-regulated an array of proangiogenic CXC chemokine genes in the NSCLC cell line A549 and in normal human tracheobronchial epithelium cells, as determined by microarray analysis. Further analysis revealed that IL-1β induced much higher protein levels of CXC chemokines in NSCLC cells than in normal human tracheobronchial epithelium cells. Con- ditioned medium from IL-1β–treated A549 cells markedly increased endothelial cell migra- tion, which was suppressed by neutralizing antibodies against CXCL5 and CXCR2. We also found that IL-1β–induced CXC chemokine gene overexpression in NSCLC cells was abro- gated with the knockdown of cyclic AMP-responsive element binding protein (CREB) or nuclear factor κB (NF-κB). Moreover, the expression of the CXC chemokine genes as well as CREB and NF-κB activities was greatly increased in the tumorigenic NSCLC cell line compared with normal, premalignant immortalized or nontumorigenic cell lines.