Impurities Removal in Seawater to Optimize the Magnesium Extraction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology
nanomaterials Article Cycle Stability and Hydration Behavior of Magnesium Oxide and Its Dependence on the Precursor-Related Particle Morphology Georg Gravogl 1,2, Christian Knoll 2,3 , Jan M. Welch 4, Werner Artner 5, Norbert Freiberger 6, Roland Nilica 6, Elisabeth Eitenberger 7, Gernot Friedbacher 7, Michael Harasek 3 , Andreas Werner 8, Klaudia Hradil 5, Herwig Peterlik 9, Peter Weinberger 2 , Danny Müller 2,* and Ronald Miletich 1 1 Department of Mineralogy and Crystallography, University of Vienna, Althanstraße 14, 1090 Vienna, Austria; [email protected] (G.G.); [email protected] (R.M.) 2 Institute of Applied Synthetic Chemistry, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (C.K.); [email protected] (P.W.) 3 Institute of Chemical, Environmental & Biological Engineering, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] 4 Atominstitut, TU Wien, Stadionallee 2, 1020 Vienna, Austria; [email protected] 5 X-ray Center, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (W.A.); [email protected] (K.H.) 6 RHI-AG, Magnesitstraße 2, 8700 Leoben, Austria; [email protected] (N.F.); [email protected] (R.N.) 7 Institute of Chemical Technologies and Analytics, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] (E.E); [email protected] (G.F.) 8 Institute for Energy Systems and Thermodynamics, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria; [email protected] 9 Faculty of Physics, University of Vienna, Boltzmanngasse 5, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-1-5880-1163-740 Received: 31 August 2018; Accepted: 2 October 2018; Published: 7 October 2018 Abstract: Thermochemical energy storage is considered as an auspicious method for the recycling of medium-temperature waste heat. -

Brno University of Technology Vysoké Učení Technické V Brně
BRNO UNIVERSITY OF TECHNOLOGY VYSOKÉ UČENÍ TECHNICKÉ V BRNĚ FACULTY OF CHEMISTRY FAKULTA CHEMICKÁ INSTITUTE OF CHEMISTRY AND TECHNOLOGY OF ENVIRONMENTAL PROTECTION ÚSTAV CHEMIE A TECHNOLOGIE OCHRANY ŽIVOTNÍHO PROSTŘEDÍ DEGRADATION OF HEAT TRANSFER FLUIDS IN THERMAL SOLAR SYSTEMS AND PROPANE-1,3-DIOL AS A NEW OPTION STÁRNUTÍ TEPLONOSNÝCH KAPALIN V TERMICKÝCH SOLÁRNÍCH SYSTÉMECH A PROPAN-1,3-DIOL JAKO NOVÁ MOŽNOST DOCTORAL THESIS DIZERTAČNÍ PRÁCE AUTHOR Ing. František Mikšík AUTOR PRÁCE SUPERVISOR prof. Ing. Josef Čáslavský, CSc. ŠKOLITEL BRNO 2018 ABSTRAKT Stárnutí teplonosných kapalin na organické bázi je dlouhodobým problémem, který je znám od počátku jejich používání. První část této disertační práce je tak věnována případové studii funkčního experimentálního systému, který byl jako nový naplněn teplonosnou kapalinou na bázi propan-1,2-diol a pozorován po období 7 let. Pro analýzu stárnutí kapaliny v tomto systému byly sledovány základní provozní vlastnosti kapaliny jako jsou hustota, viskozita, teplota tuhnutí, pH a obsah kovů. Skrze tyto vlastnosti tak bylo sledováno stárnutí kapaliny nepřímo. Přímé sledování stárnutí bylo posléze provedeno analýzou degradačních produktů, jako jsou organické kyseliny a změny ve složení směsi pomocí izotachoforézy a hmotnostní spektrometrie. Pro srovnání byly taktéž analyzovány vybrané vzorky z několik dalších systémů plněných identickou kapalinou s prokazatelně pokročilou formou degradace. V druhé části práce jsou představeny základní fyzikálně-chemické vlastnosti směsí propan-1,3-diolu s vodou a jejich analytické hodnocení a matematické modelování pro universální použití jakožto nového základu pro nemrznoucí teplonosné kapaliny. Na základě dostupných informací je pak hodnocena použitelnost této směsi. Výhoda propan-1,3-diolu je spatřována především ve výrobě z obnovitelných zdrojů a v některých fyzikálních a chemických vlastnostech, které dle dosavadních poznatků předčívají doposud používané glykolové směsi. -

The Quantitative Separation of Calcium from Magnesium. by Walterc
QUANTITATIVE SEPARATION OF CALCIUM FROM MAGNESIUM. 91 7 ium, the latter can be obtained in the least soluble portion and in this respect differs from nearly all the other methods for its separation. In conclusion, it may be as well to mention that the third part of this paper will appear shortly. We also acknowledge our indebtedness to the Welsbach Company for material supplied for future investigation through the courtesy of Dr. H. S. Miner. NEW HAMPSHIPB COLLEGE, DURHAM, N. H., June 99 1909. THE QUANTITATIVE SEPARATION OF CALCIUM FROM MAGNESIUM. BY WALTERC. BLASDALE. Received June 2, xgog. Although methods for the separation of these elements, which de- pend upon the relative solubilities of their carbonates, chromates and sulphates, respectively, have found advocates, none of these possess decided advantages over the more generally used oxalate process. The theory of the last-mentioned method has been discussed by Richards, McCaffrey and Bisbee,' who also determined the magnitude of the error resulting from the occlusion of magnesium by the precipitated calcium under a series of varying conditions. These experiments showed that this error could be greatly reduced by precipitating with oxalic acid from a slightly acid solution and subsequent neutralization, rather than by precipitating with ammonium oxalate from an alkaline solution ac- cording to the usual method. A somewhat extended use of the newer method has in general given excellent results, but as the experiments referred to were practically limited to cases in which approximately equivalent amounts of calcium and magnesium were present, and as the method seemed to be, for many practical purposes at least, unduly elab- orate, it seemed desirable to secure further experimental data with re- gard to its more general application. -

The Structure of Oxalate Decarboxylase at Its Active Ph
bioRxivRunning preprint head: doi: https://doi.org/10.1101/426874 Low pH Structure, Oxalate; this version Decarboxylase posted September 25, 2018. The copyright holder for this preprint (which was1 not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The Structure of Oxalate Decarboxylase at its Active pH M. J. Burg, J. L. Goodsell, U. T. Twahir, S. D. Bruner, and A. Angerhofer, bioRxivLow preprintpH Structure, doi: https://doi.org/10.1101/426874 Oxalate Decarboxylase; this version posted September 25, 2018. The copyright holder for this preprint (which was2 not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Oxalate decarboxylase catalyzes the redox-neutral unimolecular disproportionation reaction of oxalic acid. The pH maximum for catalysis is ~4.0 and activity is negligible above pH7. Here we report on the first crystal structure of the enzyme in its active pH range at pH4.6, and at a resolution of 1.45 Å, the highest to date. The fundamental tertiary and quaternary structure of the enzyme does not change with pH. However, the low pH crystals are heterogeneous containing both a closed and open conformation of a flexible loop region which gates access to the N-terminal active site cavity. Residue E162 in the closed conformation points away from the active-site Mn ion owing to the coordination of a buffer molecule, acetate. Since the quaternary structure of the enzyme appears unaffected by pH many conclusions drawn from the structures taken at high pH remain valid. -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

United States Patent to 11 4,018,876 Jordan 45 Apr
United States Patent to 11 4,018,876 Jordan 45 Apr. 19, 1977 54) PROCESS FOR THE PRODUCTION OF METAL OXALATES AND SODA ASH OTHER PUBLICATIONS 76 Inventor: Robert Kenneth Jordan, The Carlton Alien Property Custodian (APC) 227107, Suzuki, H., House, Suite 1431, 550 Grant St., filed Aug. 27, 1938, published Apr. 20, 1943. Pittsburgh, Pa. 15219 Primary Examiner-O. R. Vertiz 22 Filed: Mar. 4, 1975 Assistant Examiner-Gary P. Straub 21 ) Appl. No.: 555,189 57 ABSTRACT 52) U.S. C. .............. is a se a s & 8 was a 423.1421; 260/.538 A process for the simultaneous production of relatively 51 Int. Cl.................... C07C 55/06; C01B 3 1/24 insoluble metal oxalates and carbonates and bicarbon (58) Field of Search ........... 260/538,527 R, 526 R; ates of alkali metals and ammonium in which a metal 423.1419-422,430 carbonate and an ammonium or alkali metal oxalate 56) References Cited are combined in water or methanol, optionally with the UNITED STATES PATENTS addition of carbon dioxide. l,687,480 0/1928 Buchanan et al. ................. 260/.538 2,002,342 5/1935 Enderli .............................. 260/.538 3 Claims, No Drawings 4,018,876 1. 2 Even considerable ferrous carbonate is available, but PROCESS FOR THE PRODUCTION OF METAL as iron ore, FeO is very cheap, there is no interest in OXALATES AND SODA ASH it. But other valuable carbonate ores or metal carbon ate intermediates could be available if there were and This invention relates to a process for the simulta 5 inexpensive direct way to process them to useful inter neous production of metal oxalates and ammonium or mediates. -
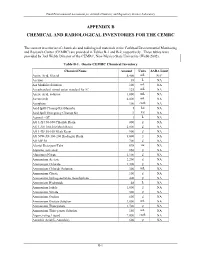
Appendix B Chemical and Radiological Inventories for the Cemrc
Final Environmental Assessment for Actinide Chemistry and Repository Science Laboratory APPENDIX B CHEMICAL AND RADIOLOGICAL INVENTORIES FOR THE CEMRC The current inventories of chemicals and radiological materials at the Carlsbad Environmental Monitoring and Research Center (CEMRC) are provided in Tables B-1 and B-2, respectively. These tables were provided by Joel Webb, Director of the CEMRC, New Mexico State University (Webb 2002). Table B-1. Onsite CEMRC Chemical Inventory Chemical Name Amount Units SARA Limit Acetic Acid, Glacial 5,400 mL NAa Acetone 38 L NA AA Modifier Solution 100 mL NA AccuStandard mixed anion standard for IC 125 mL NA Acetic Acid, solution 1,000 mL NA Acetonitrile 4,000 mL NA Acetylene 100 cu.ft. NA Acid Spill Cleanup Kit (Hazorb) 1 kit NA Acid Spill Emergency Cleanup Kit 3 kit NA Aerosol - OT 1 L NA AG 1-X4 50-100 Chloride Resin 800 g NA AG 1-X8 100-200 Mesh Resin 2,000 g NA AG 1-X8 50-100 Mesh Resin 900 g NA AG 50W-X8 100-200 Hydrogen Resin 3,000 g NA AG MP 50 700 g NA Alcojet Detergent/Tabs 698 oz NA Alumina, activated 850 g NA Aluminum Nitrate 2,100 g NA Ammonium Acetate 2,200 g NA Ammonium Chloride 1,300 g NA Ammonium Chloride Solution 300 mL NA Ammonium Citrate 100 g NA Ammonium hydrogenoxalate, hemihydrate 400 g NA Ammonium Hydroxide 48 L NA Ammonium Iodide 1,000 g NA Ammonium Nitrate 500 g NA Ammonium Oxalate 600 g NA Ammonium Oxalate Solution 2,000 mL NA Ammonium Thiocyanate 1,700 g NA Ammonium Thiocyanate Solution 150 mL NA Argon, refrig. -

(19) 11 Patent Number: 6022947
US006022947A United States Patent (19) 11 Patent Number: 6,022,947 Frihart et al. (45) Date of Patent: Feb. 8, 2000 54) LIGHT-COLORED, LOW MOLECULAR 4,391,640 7/1983 Okoshi et al.. WEIGHT PHENOLC-MODIFIED ROSIN 4,398,016 8/1983 Homma et al. ...................... 528/158.5 ESTERS 4,744,925 5/1988 Lampo et al.. 4,857,624 8/1989 DeBlasi et al. ......................... 528/129 5,162,496 11/1992 Johnson, Jr. ............................ 530/212 (75) Inventors: Charles R. Frihart, Lawrenceville, 5,177.133 1/1993 Peck et al. .............................. 524/139 N.J.; Kenneth E. Krajca; Brett A. 5,405,932 4/1995 Bender et al. .......................... 528/104 Neumann, both of Savannah, Ga. 5.830,992 11/1998 Whalen ................................... 530/215 73 Assignee: Union Camp Corporation, Wayne, OTHER PUBLICATIONS N.J. Union Camp. Uni-Rez(R) Product Data, “Uni-Rez(R 9200 Phenolic Modified Resin,” Union Camp Corporation 21 Appl. No.: 09/156,337 Chemical Products Division, Sep.1994, 2 pages. 22 Filed: Sep. 18, 1998 Primary Examiner Nathan M. Nutter (51) Int. Cl." .................................. C09F1/02; CO9F1/04 Attorney, Agent, or Firm Seed and Berry LLP 52 U.S. Cl. .......................... 530/212; 530/213; 530/215; 530/216; 530/218; 530/219; 528/86; 528/167; 57 ABSTRACT 528/171; 528/176; 528/211; 156/327; 156/335; Light-colored phenolic-modified roSin esters, Suitable for 52.5/54.4 use as, for example, tackifiers in adhesive compositions, are 58 Field of Search ..................................... 530/212, 213, prepared by reacting together rosin, a phenolic compound, 530/215, 216, 218, 219; 528/86, 167, 171, formaldehyde or a reactive equivalent thereof, and a non 176, 211; 156/327, 335; 525/54.4 phenolic hydroxyl-containing organic compound in the presence of at least one lightening agent Selected from 56) References Cited phenol Sulfide compounds, phosphorous acid, esters of U.S. -

Comprehensive Analysis of Direct Aqueous Mineral Carbonation Using
Comprehensive analysis of direct aqueous mineral carbonation using dissolution enhancing organic additives Benjamin Bonfils, Carine Julcour-Lebigue, François Guyot, Françoise Bodénan, Pierre Chiquet, Florent Bourgeois To cite this version: Benjamin Bonfils, Carine Julcour-Lebigue, François Guyot, Françoise Bodénan, Pierre Chiquet, et al.. Comprehensive analysis of direct aqueous mineral carbonation using dissolution enhancing or- ganic additives. International Journal of Greenhouse Gas Control, Elsevier, 2012, 9, pp.334-346. 10.1016/j.ijggc.2012.05.009. hal-00773507 HAL Id: hal-00773507 https://hal-brgm.archives-ouvertes.fr/hal-00773507 Submitted on 22 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/21201 Official URL: https://doi.org/10.1016/j.ijggc.2012.05.009 To cite this version: Bonfils, Benjamin and Julcour-Lebigue, Carine and Guyot, François and Bodénan, Françoise and Chiquet, Pierre and Bourgeois, Florent Comprehensive analysis of direct aqueous mineral carbonation using dissolution enhancing organic additives. (2012) International Journal of Greenhouse Gas Control, 9. -

Characterization of Intermediate Oxidation States in CO2 Activation
PC69CH10_Weber ARI 14 March 2018 12:24 Annual Review of Physical Chemistry Characterization of Intermediate Oxidation States in CO2 Activation Leah G. Dodson,1 Michael C. Thompson,2 and J. Mathias Weber2 1JILA and NIST, University of Colorado, Boulder, Colorado 80309-0440, USA; email: [email protected] 2JILA and Department of Chemistry and Biochemistry, University of Colorado, Boulder, Colorado 80309-0440, USA; email: [email protected], [email protected] ANNUAL REVIEWS Further Click here to view this article's online features: • Download figures as PPT slides • Navigate linked references • Download citations • Explore related articles • Search keywords Annu. Rev. Phys. Chem. 2018. 69:231–52 Keywords First published as a Review in Advance on carbon dioxide, formate, oxalate, carbonate, C–O activation, CO2 February 28, 2018 Annu. Rev. Phys. Chem. 2018.69:231-252. Downloaded from www.annualreviews.org reduction catalysis Access provided by University of Colorado - Boulder on 04/23/19. For personal use only. The Annual Review of Physical Chemistry is online at physchem.annualreviews.org Abstract https://doi.org/10.1146/annurev-physchem- Redox chemistry during the activation of carbon dioxide involves changing 050317-021122 the charge state in a CO2 molecular unit. However, such changes are usually Copyright c 2018 by Annual Reviews. not well described by integer formal charges, and one can think of COO All rights reserved functional units as being in intermediate oxidation states. In this article, we discuss the properties of CO2 and CO2-based functional units in various charge states. Besides covering isolated CO2 and its ions, we describe the CO2-based ionic species formate, oxalate, and carbonate. -

Magnesium Oxalate Dihydrate
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Magnesium oxalate dihydrate ≥ 99%, pure article number: 0495 date of compilation: 13.07.2017 Version: 1.0 en SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Identification of the substance Magnesium oxalate dihydrate Article number 0495 Registration number (REACH) This information is not available. Index No 607-007-00-3 EC number 208-932-1 CAS number 547-66-0 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet e-mail (competent person) : [email protected] 1.4 Emergency telephone number Emergency information service Poison Centre Munich: +49/(0)89 19240 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 3.1O acute toxicity (oral) (Acute Tox. 4) H302 3.1D acute toxicity (dermal) (Acute Tox. 4) H312 Malta (en) Page 1 / 11 Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Magnesium oxalate dihydrate ≥ 99%, pure article number: 0495 2.2 Label elements Labelling according to Regulation (EC) No 1272/2008 (CLP) Signal word Warning Pictograms Hazard statements H302+H312 Harmful if swallowed or in contact with skin Precautionary statements Precautionary statements - prevention P270 Do not eat, drink or smoke when using this product. -

Chemical Equilibrium 4
CHAPTER Chemical Equilibrium 4 ow we arrive at the point where real chemistry begins. Chemical thermo- Thermodynamic background dynamics is used to predict whether a mixture of reactants has a sponta- 4.1 The reaction Gibbs energy neous tendency to change into products, to predict the composition of the ⌬ N 4.2 The variation of rG with reaction mixture at equilibrium, and to predict how that composition will be mod- composition ified by changing the conditions. In biology, life is the avoidance of equilibrium, 4.3 Reactions at equilibrium and the attainment of equilibrium is death, but knowing whether equilibrium lies CASE STUDY 4.1: Binding of in favor of reactants or products under certain conditions is a good indication of oxygen to myoglobin and the feasibility of a biochemical reaction. Indeed, the material we cover in this chap- hemoglobin ter is of crucial importance for understanding the mechanisms of oxygen transport 4.4 The standard reaction in blood, metabolism, and all the processes going on inside organisms. Gibbs energy There is one word of warning that is essential to remember: thermodynamics is silent about the rates of reaction. All it can do is to identify whether a particular re- The response of equilibria to the conditions action mixture has a tendency to form products; it cannot say whether that ten- dency will ever be realized. We explore what determines the rates of chemical re- 4.5 The presence of a catalyst actions in Chapters 6 through 8. 4.6 The effect of temperature Coupled reactions in Thermodynamic background bioenergetics 4.7 The function of adenosine The thermodynamic criterion for spontaneous change at constant temperature and triphosphate pressure is ⌬G Ͻ 0.