Appendix B Chemical and Radiological Inventories for the Cemrc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phosphate-Based Treatments for Conservation of Stone
RILEM Technical Letters (2017) 2: 14‐19 DOI: http://dx.doi.org/10.21809/rilemtechlett.2017.34 Phosphate‐based treatments for conservation of stone Enrico Sassoni a* a Department of Civil, Chemical, Environmental and Materials Engineering, University of Bologna, Via Terracini 28, 40131, Bologna, Italy Received: 30 May 2017 / Accepted: 09 August 2017 / Published online: 9 October 2017 © The Author(s) 2017. This article is published with open access and licensed under a Creative Commons Attribution 4.0 International License. Abstract To overcome the limitations of currently available protectives and consolidants for carbonate stones (such as marble and limestone), in 2011 the use of calcium phosphate was proposed. The idea is forming calcium phosphates (ideally hydroxyapatite) as the reaction product between the substrate and an aqueous solution of a phosphate salt that the stone is treated with. In this paper, the studies aimed at identifying the best treatment conditions (in terms of nature and concentration of the phosphate precursor, solution pH, reaction time, ionic and organic additions) are first briefly summarized. Then, the efficacy of the phosphate treatment in protecting marble from dissolution in rain and restoring cohesion of weathered marble and limestone is discussed. Some recent studies on the use of the phosphate treatment on alternative substrates and some future steps for research on the topic are finally outlined. Keywords: Cultural heritage; Marble; Hydroxyapatite; Protection; Consolidation 1 Introduction improve mechanical properties, by providing a binding action between the stone grains. Organic products are A great part of cultural heritage objects (e.g. monuments, effective in improving mechanical properties, but again architectural decorations and statues) is made of carbonate they lack compatibility and durability. -

Effects on Bromine, Chlorine and SO2 Under Air Firing and Oxy-Fuel
GAS-PHASE MERCURY OXIDATION: EFFECTS OF BROMINE, CHLORINE AND SO2 UNDER AIR FIRING AND OXY-FUEL CONDITIONS, EXPERIMENTAL AND MODELING STUDY by Paula Andrea Buitrago A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemical Engineering The University of Utah August 2011 Copyright © Paula Andrea Buitrago 2011 All Rights Reserved STATEMENT OF DISSERTATION APPROVAL The dissertation of Paula Andrea Bnitrago has been approved by the following supervisory committee members: Geoffrey D. Silcox , Chair 4/1112011 Date Approved JoAnn S. Lighty , Member 4/1112011 Date Approved Jost Wendt , Member 4/18/2011 bate Approved Connie Senior , Member 4/15/2011 Date Approved Eric G. Eddings , Member 4/1112011 bate Approved and by JoAnn S. Lighty , Chair of ----------------~~~~==~~--------------- the Department of Chemical Engineering and by Charles A. Wight, Dean of The Graduate School. ABSTRACT The mercury in coal is emitted in its elemental state when the coal is burned. As the combustion flue gas cools, reactions under homogeneous and heterogeneous conditions between mercury and species such chlorine, bromine, SOx and NOx can take place. The temperature and concentration of these species determines the extent of mercury oxidation. The main objective of this study was to evaluate the effects of bromine, chlorine, SOx and NOx on gas-phase mercury oxidation reactions in flue gas. This study used a methane-fired, bench-scale reactor and CHEMKIN software for performing kinetic calculations. The model included reaction pathways to account for halogen, mercury, and combustion chemistry. The experimental results showed that chlorine is not effective as a gas-phase oxidant of mercury compared with other halogens such as bromine. -

Brno University of Technology Vysoké Učení Technické V Brně
BRNO UNIVERSITY OF TECHNOLOGY VYSOKÉ UČENÍ TECHNICKÉ V BRNĚ FACULTY OF CHEMISTRY FAKULTA CHEMICKÁ INSTITUTE OF CHEMISTRY AND TECHNOLOGY OF ENVIRONMENTAL PROTECTION ÚSTAV CHEMIE A TECHNOLOGIE OCHRANY ŽIVOTNÍHO PROSTŘEDÍ DEGRADATION OF HEAT TRANSFER FLUIDS IN THERMAL SOLAR SYSTEMS AND PROPANE-1,3-DIOL AS A NEW OPTION STÁRNUTÍ TEPLONOSNÝCH KAPALIN V TERMICKÝCH SOLÁRNÍCH SYSTÉMECH A PROPAN-1,3-DIOL JAKO NOVÁ MOŽNOST DOCTORAL THESIS DIZERTAČNÍ PRÁCE AUTHOR Ing. František Mikšík AUTOR PRÁCE SUPERVISOR prof. Ing. Josef Čáslavský, CSc. ŠKOLITEL BRNO 2018 ABSTRAKT Stárnutí teplonosných kapalin na organické bázi je dlouhodobým problémem, který je znám od počátku jejich používání. První část této disertační práce je tak věnována případové studii funkčního experimentálního systému, který byl jako nový naplněn teplonosnou kapalinou na bázi propan-1,2-diol a pozorován po období 7 let. Pro analýzu stárnutí kapaliny v tomto systému byly sledovány základní provozní vlastnosti kapaliny jako jsou hustota, viskozita, teplota tuhnutí, pH a obsah kovů. Skrze tyto vlastnosti tak bylo sledováno stárnutí kapaliny nepřímo. Přímé sledování stárnutí bylo posléze provedeno analýzou degradačních produktů, jako jsou organické kyseliny a změny ve složení směsi pomocí izotachoforézy a hmotnostní spektrometrie. Pro srovnání byly taktéž analyzovány vybrané vzorky z několik dalších systémů plněných identickou kapalinou s prokazatelně pokročilou formou degradace. V druhé části práce jsou představeny základní fyzikálně-chemické vlastnosti směsí propan-1,3-diolu s vodou a jejich analytické hodnocení a matematické modelování pro universální použití jakožto nového základu pro nemrznoucí teplonosné kapaliny. Na základě dostupných informací je pak hodnocena použitelnost této směsi. Výhoda propan-1,3-diolu je spatřována především ve výrobě z obnovitelných zdrojů a v některých fyzikálních a chemických vlastnostech, které dle dosavadních poznatků předčívají doposud používané glykolové směsi. -

Chemistry Inventory; Fall
CHEMISTRY FALL 2005 MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Aluminum 9 1.5 kg Aluminum chloride, anhydrous, 98.5% 9 0.2 kg Aluminum chloride · 6H2O 9 0.5 kg Aluminum hydroxide 9 0.5 kg Aluminum nitrate 9 0.5 kg Aluminum sulfate 9 0.5 kg Ammonia, concentrated 9 4.0 L Ammonium acetate 9 0.2 kg Ammonium chloride 9 Ammonium dihydrogen phosphate (monobasic) 9 0.4 kg J.T. Baker Ammonium hydrogen phosphate (dibasic) No 0.5 kg Ammonium nitrate 9 2.5 kg Ammonium oxalate 9 0.7 kg Ammonium peroxydisulfate 9 0.5 kg Ammonium sulfate 9 0.2 kg Antimony 9 0.4 kg Barium chloride, anhydrous 9 2.5 kg Barium chloride · 2H2O 9 2.5 kg Barium nitrate 9 0.8 kg Bismuth 9 2.0 kg Boric Acid 9 0.4 kg Brass 9 Bromine 9 2.5 kg Cadmium 9 0.1 kg Cadmium nitrate 9 0.3 kg Calcium acetate · xH2O 9 0.5 kg Calcium carbide 9 1.0 kg Calcium carbonate 9 2.2 kg Calcium chloride 9 1.0 kg Calcium hydroxide 9 0.3 kg Calcium nitrate · 4H2O 9 1.0 kg Calcium oxide 9 0.3 kg Calcium sulfate · 2H2O 9 1.0 kg Carbon 9 0.1 kg Ceric ammonium nitrate 9 0.5 kg Cesium chloride 9 0.01 kg Chromium 9 0.01 kg Chromium chloride 9 0.5 kg Chromium nitrate 9 0.5 kg Cobalt 9 0.025 kg Cobalt chloride 9 0.7 kg Cobalt nitrate 9 0.6 kg Copper (assorted) 9 4.0 kg Copper acetate 9 0.05 kg Copper chloride 9 0.1 kg Copper nitrate 9 3.5 kg Copper oxide 9 0.4 kg Cupric sulfate, anhydrous 9 0.5 kg Cupric sulfate · 5H2O 9 2.75 kg EDTA 9 0.6 kg Iodine 9 2.0 kg Iron (assorted) 9 5.0 kg MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Ferric ammonium -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12 -

The Application of Controlled Radical Polymerization Processes on the Graft Copolymerization of Hydrophobic Substituents Onto Guar Gum and Guar Gum Derivatives
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2007 The pplica ation of controlled radical polymerization processes on the graft copolymerization of hydrophobic substituents onto guar gum and guar gum derivatives Veronica Holmes Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Chemistry Commons Recommended Citation Holmes, Veronica, "The ppa lication of controlled radical polymerization processes on the graft opoc lymerization of hydrophobic substituents onto guar gum and guar gum derivatives" (2007). LSU Doctoral Dissertations. 3220. https://digitalcommons.lsu.edu/gradschool_dissertations/3220 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. THE APPLICATION OF CONTROLLED RADICAL POLYMERIZATION PROCESSES ON THE GRAFT COPOLYMERIZATION OF HYDROPHOBIC SUBSTITUENTS ONTO GUAR GUM AND GUAR GUM DERIVATIVES A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College In partial fulfillment of the Requirements for the degree of Doctor of Philosophy In The Department of Chemistry By Veronica Holmes B.S., Southern University A&M, Baton Rouge, LA, 1999 May, 2007 Acknowledgements My matriculation through graduate school would not have been possible without the support and guidance of Dr. William H. Daly. His kindness and patience during my graduate endeavors has been unprecedented. Along with the support of past and present graduate students in our research lab, I have had an excellent support system for the duration of time I have spent with this research. -

Ceric Ammonium Nitrate (CAN) Catalyzed Baeyer-Villiger Oxidation of Carbonyl Compounds, Specially 20-Oxosteroids
Indian Journal of Chemistry Vol. 43B, June 2004, pp . 1275-1281 Ceric ammonium nitrate (CAN) catalyzed Baeyer-Villiger oxidation of carbonyl compounds, specially 20-oxosteroids Papori Goswami, Saroj Hazarika, Archana M Das & Pritish Chowdhury* Natural Products Chemistry Division, Regional Research Laboratory, Jorhat 785006, India e-mail: [email protected] Received 4 February 2003; accepted (revised) 10 December 2003 The role of ceric ammonium nitrate (CAN) as an effective catalyst in the peracid induced Baeyer-Villiger oxidation of carbonyl compounds with special reference to steroids has been demonstrated. IPC: Int.C1.7 C 07 K 1/00 Ceric ammonium nitrate (CAN) finds application in temperature even when kept for more than 48 hr. Fur synthetic organic chemistry for various chemical ther, GLC experiment confirmed 40-55% conversion 2 transformations, viz., nitration 1, nitroacetamidation , of benzophenone 12 to phenyl benzoate 12a in 5 hr complex formation with various alcohols3 etc. Its role when CAN was used as a catalyst along with peracid as single electron oxidant has been reported in a num whereas only 8% conversion was observed in the ab 4 8 ber of publications including some recent reviews - . sence of CAN when kept for more than 24 hr. During CAN-induced oxidative radical transformations of our investigation, we also found that for all the cases 9 steroids have also been reported . We too have re (substrates 5-8, 10, 11, 13-15) the oxidation furni shed ported lO the catalytic action of CAN in the esterifica only one isomer viz. 5a-8a, lOa, lla, 13a-15a as con tion of carboxylic acids in very high yield . -

Quinone Formation Via Ceric Ammonium Nitrate Oxidations of 2-Alkyl-1,4-Dialkoxybenzenes
Quinone Formation via Ceric Ammonium Nitrate Oxidations of 2-Alkyl-1,4-dialkoxybenzenes by Alexander Linwood Simmons April, 2016 Director of Thesis: Brian E. Love, PhD Major Department: Chemistry Quinones are cyclohexadiendiones that have a variety of uses ranging from medical applications to synthetic building blocks.1 Medicinal applications stem from the potent biological activity (e.g. antitumor and antibiotic) these compounds and some derivatives possess.2, 3 The most common preparation method to access these compounds is oxidative demethylation of hydroquinone dimethyl ethers (1, R1=R3: Me) typically using ceric ammonium nitrate (CAN) as seen in Figure 1. Oxidation using CAN can yield a product mixture of the (mono)quinone (2) and the symmetric dimeric quinone (3). Previous work4, 5 in our group has resulted in the development of several protocols for altering the monoquinone to diquinone ratio by altering reaction conditions (e.g. substrate concentration, mode of addition, etc.). The current focus further explores manipulation of this ratio and reaction efficacy through substrate solubility and cerium coordination. We will discuss how ether linkages of various hydrophobicities and coordination modes change product outcome and if altering a single ether linkage (R1) or both linkages (both R1 and R3) affect the product ratio. 1 2 3 Figure 1 – General Substrate Reaction Quinone Formation via Ceric Ammonium Nitrate Oxidations of 2-Alkyl-1,4-dialkoxybenzenes A Thesis Presented To the Faculty of the Department of Chemistry East Carolina University In Partial Fulfillment of the Requirements for the Degree Master of Science in Chemistry by Alexander Linwood Simmons April, 2016 © Alexander Linwood Simmons, 2016 Quinone Formation via Ceric Ammonium Nitrate Oxidations of 2-Alkyl-1,4-dialkoxybenzenes by Alexander Linwood Simmons Approved by: Director of Thesis: ____________________________________________________________ Brian E. -

The Structure of Oxalate Decarboxylase at Its Active Ph
bioRxivRunning preprint head: doi: https://doi.org/10.1101/426874 Low pH Structure, Oxalate; this version Decarboxylase posted September 25, 2018. The copyright holder for this preprint (which was1 not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The Structure of Oxalate Decarboxylase at its Active pH M. J. Burg, J. L. Goodsell, U. T. Twahir, S. D. Bruner, and A. Angerhofer, bioRxivLow preprintpH Structure, doi: https://doi.org/10.1101/426874 Oxalate Decarboxylase; this version posted September 25, 2018. The copyright holder for this preprint (which was2 not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Oxalate decarboxylase catalyzes the redox-neutral unimolecular disproportionation reaction of oxalic acid. The pH maximum for catalysis is ~4.0 and activity is negligible above pH7. Here we report on the first crystal structure of the enzyme in its active pH range at pH4.6, and at a resolution of 1.45 Å, the highest to date. The fundamental tertiary and quaternary structure of the enzyme does not change with pH. However, the low pH crystals are heterogeneous containing both a closed and open conformation of a flexible loop region which gates access to the N-terminal active site cavity. Residue E162 in the closed conformation points away from the active-site Mn ion owing to the coordination of a buffer molecule, acetate. Since the quaternary structure of the enzyme appears unaffected by pH many conclusions drawn from the structures taken at high pH remain valid. -
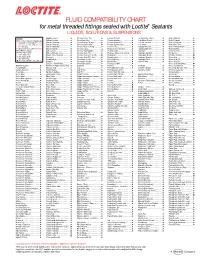
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Ammonium Phosphate on Gypsum
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by AMS Acta - Alm@DL - Università di Bologna HMC 2016 4 th Historic Mortars Conference Preliminary study on the use of ammonium phosphate for the conservation of marble-imitating gypsum-stuccoes Enrico Sassoni 1, Gabriela Graziani 2, George W. Scherer 3 and Elisa -ranzoni 4 0Tmh dqrhsxneAnknfm+)skx+ [email protected] 1Tmh dqrhsxneAnknfm+)skx+ faqhdk-fqyhmh1.tmhan-hs 2 OqhmbdsnmTmh dqrhsx+MI+TR@+ rbgdqdq.oqhmbdsnm-dct 0 Tmh dqrhsxneAnknfm+)skx+ dkhr-eqmynmh.tmhan-hs Abstract6 In this study, a novel method for consolidation and im rovement of resistance to water of gy sum-stuccoes was reliminarily investigated. The idea is treating gy sum with an aqueous solution of diammonium hydrogen hos hate (DAP, (.H 4)2HPO 4) to form hydroxya atite (HAP, Ca 10 (PO 4)6(OH) 2), which has much lower solubility than gy sum. Tests carried out on gy sum aste sam les, manufactured to resemble historic stuccoes, showed that, after treatment with the DAP solution, a significant im rovement in mechanical ro erties was achieved and brushite (CaHPO 4ì2H 2O) was formed (alongside some other by- roducts, that can be removed by an additional oultice treatment). Even if brushite is more soluble than HAP, still its formation is ex ected to be beneficial for stuccoes conservation, as brushite is significantly less soluble than gy sum. Introduction Since antiquity, gy sum-based stuccoes have been frequently used to imitate recious white or colored marbles, when the use of real marbles was not ossible, because trans ort from faraway quarries was too costly a1,2b. -

EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E)
Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Eurofins Document Reference EFGS-SOP-137-R02 Revision 2 Effective Date 6/17/2013 Status Final Historical/Local Document Number FGS-SOP-137.02 Local Document Level Level 3 Local Document Type SOP Local Document Category NA Prepared by Ryan Nelson Reviewed Dave Wunderlich and Patrick Garcia-Strickland and Approved by Revision: 2 Effective Date: 6/17/2013 Page 1 of 20 COMPANY CONFIDENTIAL. All Eurofins Frontier Global Sciences standard operating procedures contain proprietary information and are protected by Washington State Law. Proprietary information must be maintained with the strictest of confidence and must not be used or appropriated to benefit any party without prior written consent from Eurofins Frontier Global Sciences. Document Title: Eurofins Document Reference: Mercury in Water by Oxidation, Purge & Trap EFGS-SOP-137-R02 and CV-AFS (EPA Method 1631, Rev E) Table of Contents 1 Revision Log: ......................................................................................................................... 4 2 Reference: ............................................................................................................................. 4 3 Cross Reference: ................................................................................................................... 5 4 Purpose: ...............................................................................................................................