Quantum Dot Photolithography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paper 73-3 Has Been Designated As a Distinguished Paper at Display Week 2018
Distinguished Student Paper 73-3 / T. Ji Paper 73-3 has been designated as a Distinguished Paper at Display Week 2018. The full- length version of this paper appears in a Special Section of the Journal of the Society for Information Display (JSID) devoted to Display Week 2018 Distinguished Papers. This Special Section will be freely accessible until December 31, 2018 via: http://onlinelibrary.wiley.com/page/journal/19383657/homepage/display_week_2018.htm Authors that wish to refer to this work are advised to cite the full-length version by referring to its DOI: https://doi.org/10.1002/jsid.640 SID 2018 DIGEST Distinguished Student Paper 73-3 / T. Ji Tingjing Ji- Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Tingjing Ji (student), Shuang Jin, Bingwei Chen, Yucong Huang, Zijing Huang, Zinan Chen, Shuming Chen*, Xiaowei Sun Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, PR China, 518055 *Corresponding author: [email protected] Abstract the traditional patterning processes. The QLED device achieved Photolithography is a high resolution and mature patterning maximum electroluminescence intensity of 23 770 cd/m2. [13] technique which has been widely used in semiconductor industry. For display application, a pixel consists of red (R), green (G) and In this work, we use photolithography to fine pattern the QD blue (B) side-by-side sub-pixels, which thereby requires a high layers. Because it is difficult to etch the QD layer, lift-off is used resolution patterning of the light-emission layers. -

Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography
Nanofabrication 2015; 2: 19–26 Research Article Open Access Soma Biswas*, Falko Brinkmann, Michael Hirtz, Harald Fuchs Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography Abstract: We present a direct way of patterning CdSe/ lithography and photolithography, these direct techniques ZnS quantum dots by dip-pen nanolithography and do not rely on resist layers and multi-step processing, but polymer pen lithography. Mixtures of cholesterol and allow the precise deposition of ink mixtures. Spot sizes in phospholipid 1,2-dioleoyl-sn-glycero-3 phosphocholine commercially widespread inkjet printing and other related serve as biocompatible carrier inks to facilitate the transfer spotting techniques are usually in the range of 50 to 500 of quantum dots from the tips to the surface during µm [5–7], while high resolution approaches (µCP, DPN and lithography. While dip-pen nanolithography of quantum PPL) reach sub-µm features. dots can be used to achieve higher resolution and smaller Microcontact printing utilises a predesigned pattern features (approximately 1 µm), polymer pen poly(dimethylsiloxane) (PDMS) stamp that is first coated lithography is able to address intermediate pattern scales with ink and subsequently pressed onto the surface in the low micrometre range. This allows us to combine manually. A total area up to cm2 can be patterned retaining the advantages of micro contact printing in large area and a lateral resolution of approximately 100 nm [8]. Dip-pen massive parallel patterning, with the added flexibility in nanolithography (Figure 1a) employs an atomic force pattern design inherent in the DPN technique. microscopy tip (AFM) as a quill pen. -
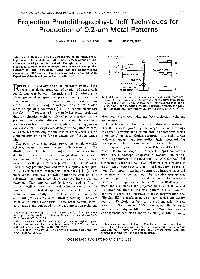
Projection Photolithography-Liftoff Techniques for Production of 0.2-Pm Metal Patterns
IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. ED-28, NO. 11, NOVEMBER 1981 1375 Projection Photolithography-Liftoff Techniques for Production of 0.2-pm Metal Patterns MARK D. FEUER AND DANIEL E. PROBER, MEMBER, IEEE GLASS FILTER Abstract-A technique whichallows the useof projection photo- \ yPHOTORESIST lithography with the photoresist liftoff process, for fabrication of sub- micrometer metal patterns, is described. Through-the-substrate (back- \II a projection) exposure of the photoresist produces the undercut profiles necessary forliftoff processing. Metal lines andsuperconducting microbridges of 0.2-pm width have been fabricated with this technique. I Experimental details and process limits are discussed. II OBJECTIVE IMMERSION OIL ECENT DEVELOPMENTS inmicrolithography have SUBSTRATE - (d) R made possible the production ofa variety of devices with PHOTORESIST %- I-l submicrometer (submicron) dimensions [ 11 , [2] , offering the (a) advantages of higherspeed and packing density.For many Fig. 1. Schematicdiagram of back-projectionand metal-liftoff pro- Josephson-effect devices in particular, submicron dimensions cedure. (a) Exposure system, employing a Zeiss optical microscope. Image of the mask is projected through the substrate, which is shown are essential for achieving optimalperformance over awide in sideview. (b) Schematic contours of constant exposure intensity. range of operatingconditions [3] . Forsubmicron pattern (c) After photoresist development and metallization. (d) After liftoff. transfer, liftoff processing [ 11 generally -

And Nanolithography Techniques and Their Applications
Review on Micro- and Nanolithography Techniques and their Applications Alongkorn Pimpin* and Werayut Srituravanich** Department of Mechanical Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand E-mail: [email protected]*, [email protected]** Abstract. This article reviews major micro- and nanolithography techniques and their applications from commercial micro devices to emerging applications in nanoscale science and engineering. Micro- and nanolithography has been the key technology in manufacturing of integrated circuits and microchips in the semiconductor industry. Such a technology is also sparking revolutionizing advancements in nanotechnology. The lithography techniques including photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography and scanning probe lithography are discussed. Furthermore, their applications are summarized into four major areas: electronics and microsystems, medical and biotech, optics and photonics, and environment and energy harvesting. Keywords: Nanolithography, photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography, scanning probe lithography, dip-pen lithography, microsystems, MEMS, nanoscience, nanotechnology, nano-engineering. ENGINEERING JOURNAL Volume 16 Issue 1 Received 18 August 2011 Accepted 8 November Published 1 January 2012 Online at http://www.engj.org DOI:10.4186/ej.2012.16.1.37 DOI:10.4186/ej.2012.16.1.37 1. Introduction For decades, micro- and nanolithography technology has been contributed to the manufacturing of integrated circuits (ICs) and microchips. This advance in the semiconductor and IC industry has led to a new paradigm of the information revolution via computers and the internet. Micro- and nanolithography is the technology that is used to create patterns with a feature size ranging from a few nanometers up to tens of millimeters. -

Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
micromachines Review Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems Jessica Ortigoza-Diaz 1, Kee Scholten 1 ID , Christopher Larson 1 ID , Angelica Cobo 1, Trevor Hudson 1, James Yoo 1 ID , Alex Baldwin 1 ID , Ahuva Weltman Hirschberg 1 and Ellis Meng 1,2,* 1 Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA; [email protected] (J.O.-D.); [email protected] (K.S.); [email protected] (C.L.); [email protected] (A.C.); [email protected] (T.H.); [email protected] (J.Y.); [email protected] (A.B.); [email protected] (A.W.H.) 2 Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA * Correspondence: [email protected]; Tel.: +1-213-740-6952 Received: 31 July 2018; Accepted: 18 August 2018; Published: 22 August 2018 Abstract: Parylene C is a promising material for constructing flexible, biocompatible and corrosion- resistant microelectromechanical systems (MEMS) devices. Historically, Parylene C has been employed as an encapsulation material for medical implants, such as stents and pacemakers, due to its strong barrier properties and biocompatibility. In the past few decades, the adaptation of planar microfabrication processes to thin film Parylene C has encouraged its use as an insulator, structural and substrate material for MEMS and other microelectronic devices. However, Parylene C presents unique challenges during microfabrication and during use with liquids, especially for flexible, thin film electronic devices. In particular, the flexibility and low thermal budget of Parylene C require modification of the fabrication techniques inherited from silicon MEMS, and poor adhesion at Parylene-Parylene and Parylene-metal interfaces causes device failure under prolonged use in wet environments. -

Fundimentals of Photolithography
FUNDIMENTALS OF PHOTOLITHOGRAPHY One of the most widely used methods for creating nanoscale circuit components is Photolithography. The word lithography is derived from the Greek words lithos (stone) and graphein (to write) and finds its roots in a process invented by Aloys Senefelder in 1796. By treating a piece of limestone with certain chemicals, Senefelder was then able to transfer an image carved into the stone onto a piece of paper. This was done by coating certain parts of the porous limestone with a water repellant substance. When ink was applied to the stone it would only adhere to the untreated hydrophilic areas, and hence the image carved into the rock could be transferred repeatedly onto paper. Senefelder's technique is still used in some artistry applications today. As time progressed and technology improved, lithography methods evolved. In the 1820's a French scientist by the name of Nicephore Niepce developed the first photoresist, a component fundamental to photolithography. A photoresist is a substance that undergoes a chemical reaction when it is eXposed to light. Niepce's photoresist was a material known as Bitumen of Judea, a kind of naturally occurring asphalt. A sheet of stone, metal, or glass was coated with a thin layer of this bitumen, which became less soluble where it was eXposed to light. Areas that were uneXposed could then be removed using a solvent, and the resultant exposed areas of the sheet were etched using a chemical bath. After the remaining photoresist was removed, the sheet could then be used as a printing plate. Photolithography today is in many ways similar to the original process invented by Niepce. -

Photolithography for the Investigation of Nanostructures
Photolithography for the Investigation of Nanostructures ---------------------------------------------------------- A Thesis Presented to the Honors Tutorial College, Ohio University ---------------------------------------------------------- In Partial Fulfillment of the Requirements for Graduation from the Honors Tutorial College with the degree of Bachelor of Science in Physics ---------------------------------------------------------- Helen M. Cothrel April 2015 This thesis has been approved by the Honors Tutorial College and the Department of Physics and Astronomy ________________________________________________ Dr. Eric Stinaff Professor, Physics and Astronomy Thesis adviser ________________________________________________ Dr. David Drabold Distinguished Professor, Physics and Astronomy Honors Tutorial College Director of Studies, Physics and Astronomy ________________________________________________ Dr. Jeremy Webster Dean, Honors Tutorial College 2 Table of Contents List of Figures ................................................................................................................. 4 Abstract ........................................................................................................................... 8 Introduction and Background ....................................................................................... 10 Photolithography ....................................................................................................... 11 Quantum Dots .......................................................................................................... -

Molecular Scale Imaging with a Smooth Superlens
Molecular Scale Imaging with a Smooth Superlens Pratik Chaturvedi1, Wei Wu2, VJ Logeeswaran3, Zhaoning Yu2, M. Saif Islam3, S.Y. Wang2, R. Stanley Williams2, & Nicholas Fang1* 1Department of Mechanical Science & Engineering, University of Illinois at Urbana- Champaign, 1206 W. Green St., Urbana, IL 61801, USA. 2Information & Quantum Systems Lab, Hewlett-Packard Laboratories, 1501 Page Mill Rd, MS 1123, Palo Alto, CA 94304, USA. 3Department of Electrical & Computer Engineering, Kemper Hall, University of California at Davis, One Shields Ave, Davis, CA 95616, USA. * Corresponding author Email: [email protected] RECEIVED DATE Abstract We demonstrate a smooth and low loss silver (Ag) optical superlens capable of resolving features at 1/12th of the illumination wavelength with high fidelity. This is made possible by utilizing state-of-the-art nanoimprint technology and intermediate 1 wetting layer of germanium (Ge) for the growth of flat silver films with surface roughness at sub-nanometer scales. Our measurement of the resolved lines of 30nm half-pitch shows a full-width at half-maximum better than 37nm, in excellent agreement with theoretical predictions. The development of this unique optical superlens lead promise to parallel imaging and nanofabrication in a single snapshot, a feat that are not yet available with other nanoscale imaging techniques such as atomic force microscope or scanning electron microscope. λ = 380nm 250nm The resolution of optical images has historically been constrained by the wavelength of light, a well known physical law which is termed as the diffraction limit. Conventional optical imaging is only capable of focusing the propagating components from the source. The evanescent components which carry the subwavelength information exponentially decay in a medium with positive permittivity (ε), and positive permeability (µ) and hence, are lost before making it to the image plane. -

Nanolithography
Chapter 8 Nanolithography Gunasekaran Venugopal and Sang-Jae Kim Additional information is available at the end of the chapter http://dx.doi.org/10.5772/55527 1. Introduction Nanolithography is the branch of nanotechnology concerned with the study and application of the nanofabrication of nanometer-scale structures, meaning nanopatterning with at least one lateral dimension between the size of an individual atom and approximately 100 nm. The term nanolithography is derived from the Greek words “nanos”, meaning dwarf; “lithos”, meaning rock or stone; and “graphein” meaning to write. Therefore the literal translation is "tiny writing on stone", however nowadays one understands something different whenever this term is associated with nanotechnology. Nanolithography is used e.g. during the nano‐ fabrication of leading-edge semiconductor integrated circuits (nanocircuitry), for nanoelec‐ tromechanical systems (NEMS) or for almost any other fundamental application across various scientific disciplines in nanoresearch. This technology can be suitable to use in nanofabrication of various semiconducting Integrated Circuits (ICs), NEMS and for various applications in research. The modification in semicon‐ ductor chips at the nano-scale (in the range of 10-9 meter) is also possible. This method is contrasting to various existing nanolithographic techniques like Photolithography (Venugo‐ pal, 2011), Nanoimprint lithography (NIL), Scanning Probe Lithography (SPL), Atomic Force Microscope (AFM) nanolithography, Extreme Ultraviolet Lithography (EUVL) and X-ray Lithography. In this chapter, the various nanolithographic fabrication techniques will be discussed in detail in which we will focus the various nano-patterning techniques/procedures suitable for device fabrication and their engineering applications. This technique is mainly used for nanofabrica‐ tion. -

Colloidal Quantum Dot Photodiodes with Ligand-Dependable and Photolithographic Patterning Technique
Appl. Sci. Converg. Technol. 28(3): 55-59 (2019) https://doi.org/10.5757/ASCT.2019.28.3.55 Research Paper Colloidal Quantum Dot Photodiodes with Ligand-dependable and Photolithographic Patterning Technique ShinYoung Jeonga,b, Soon-kyu Chaa,c, and Il Ki Hana,* aNanophotonics Research Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea bSchool of Electrical Engineering, Korea University, Seoul 02841, Republic of Korea cDepartment of Converging Science and Technology, Kyunghee University, Seoul 02453, Republic of Korea Received May 13, 2019; revised May 23, 2019; accepted May 24, 2019 Abstract We patterned a quantum dot (QD) layer via photolithography and fabricated a QD photodiode using an inverted QD-light emitting diode (LED) structure. It shows behavior similar to normal photodiodes in terms of photoresponse. However, the dark current was reduced by 1/10 and the photocurrent increased 10 times in comparison with unpatterned QD photodiodes. This study is beneficial for creating multicolor QD sensors and simultaneously integrating LEDs and photosensors on the same structure. It means that the sensor works while blinking time of LED and it could be applied to advanced display technique. Keywords: Colloidal quantum dot, Patterning, Photodiodes, Photolithography, Layer-by- Layer self-assembly I. Introduction length. However, there have been few studies on broad- band photodetection using QDs. Recently, a lamination Many studies have focused on the ability to detect method has been proposed for broadband detection light over a broad spectral range for use in several [11]. However, there is a concern that signals can be technological applications, including imaging, sensing, mixed and QDs of different sizes will be quenched si- spectroscopy, and communication [1-4]. -

A Simulation Model to Characterize Photolithography Process of a Semiconductor Wafer Fabrication
Technological University Dublin ARROW@TU Dublin Articles School of Marketing 2003 A Simulation Model to Characterize Photolithography Process of a Semiconductor Wafer Fabrication Amr Arisha Technological University Dublin, [email protected] Paul Young Dublin City University, [email protected] Mohie El Baradie Dublin City University, [email protected] Follow this and additional works at: https://arrow.tudublin.ie/buschmarart Part of the Operations Research, Systems Engineering and Industrial Engineering Commons Recommended Citation Arisha, A., Young, P., El Baradie, M.: A Simulation Model to Characterize Photolithography Process of a Semiconductor Wafer Fabrication. Journal of Materials Processing Technology. 2003. This Article is brought to you for free and open access by the School of Marketing at ARROW@TU Dublin. It has been accepted for inclusion in Articles by an authorized administrator of ARROW@TU Dublin. For more information, please contact [email protected], [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 4.0 License Funder: Intel - Ireland A SIMULATION MODEL TO CHARACTERIZE PHOTOLITHOGRAPHY PROCESS OF A SEMICONDUCTOR WAFER FABRICATION A. Arisha1, P. Young1, and M. El Baradie1 1 School of Mechanical and Manufacturing Engineering, Dublin City University, Ireland; email: [email protected] ABSTRACT The pressures on semiconductor manufacturers due to cost considerations, rapid growth of process technology, quality constraints, feature size reduction, and increasingly complex products are requiring ever higher efficiency from manufacturing facilities. The complexity of manufacturing high capacity semiconductor devices means that it is impossible to analyze the process control parameters and the production configurations using traditional analytical models. -

Advanced Oxidation Scanning Probe Lithography Yu K. Ryu and Ricardo
Advanced oxidation Scanning Probe Lithography Yu K. Ryu* and Ricardo Garcia** Instituto de Ciencia de Materiales de Madrid, CSIC, c/ Sor Juana Inés de la Cruz 3, 28049 Madrid, Spain *Present address: IBM Zurich Research Laboratory, Säumerstrasse 4, 8803 Rüschlikon, Switzerland ** email address: [email protected] Abstract Force microscopy enables a variety of approaches to manipulate and/or modify surfaces. Few of those methods have evolved into advanced probe-based lithographies. Oxidation scanning probe lithography (o-SPL) is the only lithography that enables the direct and resist-less nanoscale patterning of a large variety of materials, from metals to semiconductors; from self-assembled monolayers to biomolecules. Oxidation SPL has also been applied to develop sophisticated electronic and nanomechanical devices such as quantum dots, quantum point contacts, nanowire transistors or mechanical resonators. Here, we review the principles, instrumentation aspects and some device applications of o-SPL. Our focus is to provide a balanced view of the method that introduces the key steps in its evolution, provides some detailed explanations on its fundamentals and presents current trends and applications. To illustrate the capabilities and potential of o- SPL as an alternative lithography we have favored the most recent and updated contributions in nanopatterning and device fabrication. 1 1. From atomic scale modifications to oxidation scanning probe lithography The potential of scanning probe microscopes to modify or manipulate surfaces at atomic and nanometer scales was quickly recognized after the invention of the scanning tunneling microscope (STM) [1]. The combination of nanoscale patterning with the intrinsic metrology provided by probe microscopy generated an intense scientific activity.