Advanced Oxidation Scanning Probe Lithography Yu K. Ryu and Ricardo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nanolithography Activity in the Group: BG Group and AKR Group Has Extensive Activities in the Area of Nanolithography
Nanolithography activity in the group: BG group and AKR group has extensive activities in the area of nanolithography. The work is done in a Clean room of the centre which has been established with support from the Nanomission Projects. The clean room is class 10,000 class room which is maintained at class 1000 in specific areas. The facilities in the clean room allow optical lithography, electron beam lithography (EBL) and focused ion beam (FIB) lithography. Visit of the Honourable Union Minister for Science and Technology and Earth rd Science to the Bose Centre Clean room on 3 May, 2015 (Standing in front of Helios machine, 2nd from left ) One of the activities that is carried out on a routine basis is to integrate sub- 100nm nanowire of any material produced by bottom-up approach like chemical route or physical/chemical vapour deposition to a single nanowire device connected to 2 or 4 probes. Integrating the rich materials base of bottom-up approach with nano-lithograhic process is regularly done . For attaching nanowires to prefabricated contact pads for opt-electronic or electronic measurements in addition to EBL –lift off, FIB or Focused electron beam deposited metals (Pt or W) are also used. The group has done extensive work in the area of interface physics. Cross-sectional lamella using ion-beam lithographic technique is done on regular basis. WO 3/Pt/Si 30 µµµm TEM Lamella preparation of a nanowire grown Omni probe lifting off the sample on substrate using Ion-beam lithography Interface analysis of X-TEM Specimen: Partially/non aligned NWs- Interface cross section c Ankita Ghatak, Samik Roy Moulik Barnali Ghosh, RSC Adv. -

Paper 73-3 Has Been Designated As a Distinguished Paper at Display Week 2018
Distinguished Student Paper 73-3 / T. Ji Paper 73-3 has been designated as a Distinguished Paper at Display Week 2018. The full- length version of this paper appears in a Special Section of the Journal of the Society for Information Display (JSID) devoted to Display Week 2018 Distinguished Papers. This Special Section will be freely accessible until December 31, 2018 via: http://onlinelibrary.wiley.com/page/journal/19383657/homepage/display_week_2018.htm Authors that wish to refer to this work are advised to cite the full-length version by referring to its DOI: https://doi.org/10.1002/jsid.640 SID 2018 DIGEST Distinguished Student Paper 73-3 / T. Ji Tingjing Ji- Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Tingjing Ji (student), Shuang Jin, Bingwei Chen, Yucong Huang, Zijing Huang, Zinan Chen, Shuming Chen*, Xiaowei Sun Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, PR China, 518055 *Corresponding author: [email protected] Abstract the traditional patterning processes. The QLED device achieved Photolithography is a high resolution and mature patterning maximum electroluminescence intensity of 23 770 cd/m2. [13] technique which has been widely used in semiconductor industry. For display application, a pixel consists of red (R), green (G) and In this work, we use photolithography to fine pattern the QD blue (B) side-by-side sub-pixels, which thereby requires a high layers. Because it is difficult to etch the QD layer, lift-off is used resolution patterning of the light-emission layers. -

Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography
Nanofabrication 2015; 2: 19–26 Research Article Open Access Soma Biswas*, Falko Brinkmann, Michael Hirtz, Harald Fuchs Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography Abstract: We present a direct way of patterning CdSe/ lithography and photolithography, these direct techniques ZnS quantum dots by dip-pen nanolithography and do not rely on resist layers and multi-step processing, but polymer pen lithography. Mixtures of cholesterol and allow the precise deposition of ink mixtures. Spot sizes in phospholipid 1,2-dioleoyl-sn-glycero-3 phosphocholine commercially widespread inkjet printing and other related serve as biocompatible carrier inks to facilitate the transfer spotting techniques are usually in the range of 50 to 500 of quantum dots from the tips to the surface during µm [5–7], while high resolution approaches (µCP, DPN and lithography. While dip-pen nanolithography of quantum PPL) reach sub-µm features. dots can be used to achieve higher resolution and smaller Microcontact printing utilises a predesigned pattern features (approximately 1 µm), polymer pen poly(dimethylsiloxane) (PDMS) stamp that is first coated lithography is able to address intermediate pattern scales with ink and subsequently pressed onto the surface in the low micrometre range. This allows us to combine manually. A total area up to cm2 can be patterned retaining the advantages of micro contact printing in large area and a lateral resolution of approximately 100 nm [8]. Dip-pen massive parallel patterning, with the added flexibility in nanolithography (Figure 1a) employs an atomic force pattern design inherent in the DPN technique. microscopy tip (AFM) as a quill pen. -

Bilayer, Nanoimprint Lithography Brian Faircloth Nuvonyx, Inc., Bridgeton, Missouri 63044 Henry Rohrs Washington University, St
Bilayer, nanoimprint lithography Brian Faircloth Nuvonyx, Inc., Bridgeton, Missouri 63044 Henry Rohrs Washington University, St. Louis, Missouri 63130 Richard Tiberio Cornell University, Ithaca, New York 14853 Rodney Ruoff Washington University, St. Louis, Missouri 63130 Robert R. Krchnaveka) Rowan University, Glassboro, New Jersey 08028 ͑Received 3 May 1999; accepted 21 April 2000͒ Nanoimprint lithography has been shown to be a viable means of patterning polymer films in the sub-100 nm range. In this work, we demonstrate the use of a bilayer resist to facilitate the metal liftoff step in imprinter fabrication. The bilayer resist technology exhibits more uniform patterns and fewer missing features than similar metal nanoparticle arrays fabricated with single layer resist. The bilayer resist relies upon the differential solubility between poly͑methyl methacrylate͒ and poly͑methyl methacrylate methacrylic acid copolymer͒. Evidence is presented that shows the technique has a resolution of better than 10 nm. © 2000 American Vacuum Society. ͓S0734-211X͑00͒03104-8͔ I. INTRODUCTION tion demonstrated in the polymer resist layer. The formation of patterned metal layers is one application. Finely patterned Due to the inevitable transition from the microelectronic metal layers are used as interconnects in integrated circuits. to the nanoelectronic age, the demand for sub-100 nm fea- They can also be used as catalysts for subsequent layer ture sizes in lithographic techniques will increase greatly. As growth. If the subsequent metal layers cannot readily be current devices rapidly approach the 100 nm barrier, the mi- etched, e.g., due to crystalline dependent etching rates, an croelectronics industry is considering several technologies to additive approach such as liftoff is desirable. -

Electron Beam Lithography for Nanofabrication
Departament de Física, Facultat de Ciències Universitat Autònoma de Barcelona January 2008 ElectronElectron beambeam lithographylithography forfor NanofabricationNanofabrication PhD Thesis by Gemma Rius Suñé Directed by Francesc Pérez-Murano and Joan Bausells Institut de Microelectrònica de Barcelona -------------------------------------------- The cover image corresponds to a PMMA residual found after the stripping of the resist layer. Even though it seems a new planet, it is 1µm in diameter. -------------------------------------------- This memory reflects part of the work performed at the Nanofabrication Laboratory of the IMB–CNM during the past 5 years, based on Electron Beam Lithography (EBL). Nanofabrication is a very active area of research, as can be noticed from the number of publications that appear continuously and from the number of running R&D projects. Most of the work is realized in the framework of three European research projects. Novopoly project deals with the development of new polymer materials for applications in micro and nano systems. The development of a new EBL resist is framed in this project. Within NaPa, Emerging Nanopatterning methods, the development of NEMS fabrication with EBL is used to realise discrete nanomechanical devices. They are used to characterize the performance of resonating nanostructures and signal enhancement is achieved by their integration in CMOS circuits. The aim of Charpan is the development of a new patterning tool based on several charged particle species. The incidence of charged particle beams on devices is studied to evaluate potential effects induced during fabrication. Carbon nanotube (CNT) based devices contribute to some tasks of national projects Crenatun and Sensonat. In particular, the technology for fabrication of high performance CNT field-effect transistors and their preparation for sensing applications is established. -
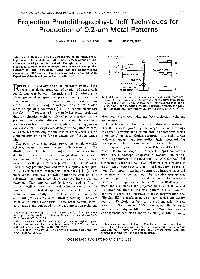
Projection Photolithography-Liftoff Techniques for Production of 0.2-Pm Metal Patterns
IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. ED-28, NO. 11, NOVEMBER 1981 1375 Projection Photolithography-Liftoff Techniques for Production of 0.2-pm Metal Patterns MARK D. FEUER AND DANIEL E. PROBER, MEMBER, IEEE GLASS FILTER Abstract-A technique whichallows the useof projection photo- \ yPHOTORESIST lithography with the photoresist liftoff process, for fabrication of sub- micrometer metal patterns, is described. Through-the-substrate (back- \II a projection) exposure of the photoresist produces the undercut profiles necessary forliftoff processing. Metal lines andsuperconducting microbridges of 0.2-pm width have been fabricated with this technique. I Experimental details and process limits are discussed. II OBJECTIVE IMMERSION OIL ECENT DEVELOPMENTS inmicrolithography have SUBSTRATE - (d) R made possible the production ofa variety of devices with PHOTORESIST %- I-l submicrometer (submicron) dimensions [ 11 , [2] , offering the (a) advantages of higherspeed and packing density.For many Fig. 1. Schematicdiagram of back-projectionand metal-liftoff pro- Josephson-effect devices in particular, submicron dimensions cedure. (a) Exposure system, employing a Zeiss optical microscope. Image of the mask is projected through the substrate, which is shown are essential for achieving optimalperformance over awide in sideview. (b) Schematic contours of constant exposure intensity. range of operatingconditions [3] . Forsubmicron pattern (c) After photoresist development and metallization. (d) After liftoff. transfer, liftoff processing [ 11 generally -

Large-Area Nanoimprint Lithography and Applications
Chapter 3 Large-Area Nanoimprint Lithography and Applications Hongbo Lan Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.72860 Abstract Large-area nanoimprint lithography (NIL) has been regarded as one of the most prom- ising micro/nano-manufacturing technologies for mass production of large-area micro/ nanoscale patterns and complex 3D structures and high aspect ratio features with low cost, high throughput, and high resolution. That opens the door and paves the way for many commercial applications not previously conceptualized or economically feasible. Great progresses in large-area nanoimprint lithography have been achieved in recent years. This chapter mainly presents a comprehensive review of recent advances in large- area NIL processes. Some promising solutions of large-area NIL and emerging methods, which can implement mass production of micro-and nanostructures over large areas on various substrates or surfaces, are described in detail. Moreover, numerous industrial- level applications and innovative products based on large-area NIL are also demon- strated. Finally, prospects, challenges, and future directions for industrial scale large- area NIL are addressed. An infrastructure of large-area nanoimprint lithography is proposed. In addition, some recent progresses and research activities in large-area NIL suitable for high volume manufacturing environments from our Labs are also intro- duced. This chapter may provide a reference and direction for the further explorations and studies of large-area micro/nanopatterning technologies. Keywords: large-area nanoimprint lithography, large-area micro/nanopatterning, full wafer NIL, roller-type NIL, roll-to-plate NIL, roll-to-roll NIL 1. -

SPM Nanolithography Workshop
NISTIR 7040 Workshop Summary Report: Scanning Probe Nanolithography Workshop A workshop sponsored by the Precision Engineering Division, Manufacturing Engineering Laboratory, NIST, under a Research Cooperation Agreement on advanced lithography of functional nanostructures signed in 2001 by the National Microelectronics Center of Spain (CNM), the National Institute of Advanced Industrial Science & Technology (AIST) of Japan, and NIST. John A. Dagata National Institute of Standards & held at Technology NIST, Gaithersburg MD 20899 Hiroshi Yokoyama on National Institute of Advanced November 12 – 13, 2002 Industrial Science & Technology of Japan Francesc Perez-Murano National Microelectronics Center of Spain U.S. DEPARTMENT OF COMMERCE Technology Administration National Institute of Standards & Technology Manufacturing Engineering Laboratory Gaithersburg MD 20899 January 2003 2 EXECUTIVE SUMMARY A workshop on Scanning Probe Microscope (SPM)-based Nanolithography was held at NIST Gaithersburg on November 24-25, 2002. The meeting was sponsored by the Precision Engineering Division, Manufacturing Engineering Laboratory, NIST, under a Research Cooperation Agreement on advanced lithography of functional nanostructures signed in 2001 by the National Microelectronics Center of Spain (CNM), the National Institute of Advanced Industrial Science & Technology (AIST) of Japan, and NIST. The workshop program focused on fundamental studies in nanoscience, the kinetics and modeling of SPM oxidation, and applications to nanotechnology, Fundamental studies included discussions of current measurement during SPM oxidation, measurement of charge & density variation in SPM oxides, control & understanding of the meniscus shape, and 3-D multiphysics modeling of electrostatics, transport, and chemical reaction during SPM oxidation. Applications to nanotechnology included the fabrication of nano-electronics, nano-photonics, nano- electromechanical, and microfluidic devices and systems. -

And Nanolithography Techniques and Their Applications
Review on Micro- and Nanolithography Techniques and their Applications Alongkorn Pimpin* and Werayut Srituravanich** Department of Mechanical Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand E-mail: [email protected]*, [email protected]** Abstract. This article reviews major micro- and nanolithography techniques and their applications from commercial micro devices to emerging applications in nanoscale science and engineering. Micro- and nanolithography has been the key technology in manufacturing of integrated circuits and microchips in the semiconductor industry. Such a technology is also sparking revolutionizing advancements in nanotechnology. The lithography techniques including photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography and scanning probe lithography are discussed. Furthermore, their applications are summarized into four major areas: electronics and microsystems, medical and biotech, optics and photonics, and environment and energy harvesting. Keywords: Nanolithography, photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography, scanning probe lithography, dip-pen lithography, microsystems, MEMS, nanoscience, nanotechnology, nano-engineering. ENGINEERING JOURNAL Volume 16 Issue 1 Received 18 August 2011 Accepted 8 November Published 1 January 2012 Online at http://www.engj.org DOI:10.4186/ej.2012.16.1.37 DOI:10.4186/ej.2012.16.1.37 1. Introduction For decades, micro- and nanolithography technology has been contributed to the manufacturing of integrated circuits (ICs) and microchips. This advance in the semiconductor and IC industry has led to a new paradigm of the information revolution via computers and the internet. Micro- and nanolithography is the technology that is used to create patterns with a feature size ranging from a few nanometers up to tens of millimeters. -

Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
micromachines Review Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems Jessica Ortigoza-Diaz 1, Kee Scholten 1 ID , Christopher Larson 1 ID , Angelica Cobo 1, Trevor Hudson 1, James Yoo 1 ID , Alex Baldwin 1 ID , Ahuva Weltman Hirschberg 1 and Ellis Meng 1,2,* 1 Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA; [email protected] (J.O.-D.); [email protected] (K.S.); [email protected] (C.L.); [email protected] (A.C.); [email protected] (T.H.); [email protected] (J.Y.); [email protected] (A.B.); [email protected] (A.W.H.) 2 Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA * Correspondence: [email protected]; Tel.: +1-213-740-6952 Received: 31 July 2018; Accepted: 18 August 2018; Published: 22 August 2018 Abstract: Parylene C is a promising material for constructing flexible, biocompatible and corrosion- resistant microelectromechanical systems (MEMS) devices. Historically, Parylene C has been employed as an encapsulation material for medical implants, such as stents and pacemakers, due to its strong barrier properties and biocompatibility. In the past few decades, the adaptation of planar microfabrication processes to thin film Parylene C has encouraged its use as an insulator, structural and substrate material for MEMS and other microelectronic devices. However, Parylene C presents unique challenges during microfabrication and during use with liquids, especially for flexible, thin film electronic devices. In particular, the flexibility and low thermal budget of Parylene C require modification of the fabrication techniques inherited from silicon MEMS, and poor adhesion at Parylene-Parylene and Parylene-metal interfaces causes device failure under prolonged use in wet environments. -

Fundimentals of Photolithography
FUNDIMENTALS OF PHOTOLITHOGRAPHY One of the most widely used methods for creating nanoscale circuit components is Photolithography. The word lithography is derived from the Greek words lithos (stone) and graphein (to write) and finds its roots in a process invented by Aloys Senefelder in 1796. By treating a piece of limestone with certain chemicals, Senefelder was then able to transfer an image carved into the stone onto a piece of paper. This was done by coating certain parts of the porous limestone with a water repellant substance. When ink was applied to the stone it would only adhere to the untreated hydrophilic areas, and hence the image carved into the rock could be transferred repeatedly onto paper. Senefelder's technique is still used in some artistry applications today. As time progressed and technology improved, lithography methods evolved. In the 1820's a French scientist by the name of Nicephore Niepce developed the first photoresist, a component fundamental to photolithography. A photoresist is a substance that undergoes a chemical reaction when it is eXposed to light. Niepce's photoresist was a material known as Bitumen of Judea, a kind of naturally occurring asphalt. A sheet of stone, metal, or glass was coated with a thin layer of this bitumen, which became less soluble where it was eXposed to light. Areas that were uneXposed could then be removed using a solvent, and the resultant exposed areas of the sheet were etched using a chemical bath. After the remaining photoresist was removed, the sheet could then be used as a printing plate. Photolithography today is in many ways similar to the original process invented by Niepce. -

Photolithography for the Investigation of Nanostructures
Photolithography for the Investigation of Nanostructures ---------------------------------------------------------- A Thesis Presented to the Honors Tutorial College, Ohio University ---------------------------------------------------------- In Partial Fulfillment of the Requirements for Graduation from the Honors Tutorial College with the degree of Bachelor of Science in Physics ---------------------------------------------------------- Helen M. Cothrel April 2015 This thesis has been approved by the Honors Tutorial College and the Department of Physics and Astronomy ________________________________________________ Dr. Eric Stinaff Professor, Physics and Astronomy Thesis adviser ________________________________________________ Dr. David Drabold Distinguished Professor, Physics and Astronomy Honors Tutorial College Director of Studies, Physics and Astronomy ________________________________________________ Dr. Jeremy Webster Dean, Honors Tutorial College 2 Table of Contents List of Figures ................................................................................................................. 4 Abstract ........................................................................................................................... 8 Introduction and Background ....................................................................................... 10 Photolithography ....................................................................................................... 11 Quantum Dots ..........................................................................................................