Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paper 73-3 Has Been Designated As a Distinguished Paper at Display Week 2018
Distinguished Student Paper 73-3 / T. Ji Paper 73-3 has been designated as a Distinguished Paper at Display Week 2018. The full- length version of this paper appears in a Special Section of the Journal of the Society for Information Display (JSID) devoted to Display Week 2018 Distinguished Papers. This Special Section will be freely accessible until December 31, 2018 via: http://onlinelibrary.wiley.com/page/journal/19383657/homepage/display_week_2018.htm Authors that wish to refer to this work are advised to cite the full-length version by referring to its DOI: https://doi.org/10.1002/jsid.640 SID 2018 DIGEST Distinguished Student Paper 73-3 / T. Ji Tingjing Ji- Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Tingjing Ji (student), Shuang Jin, Bingwei Chen, Yucong Huang, Zijing Huang, Zinan Chen, Shuming Chen*, Xiaowei Sun Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, PR China, 518055 *Corresponding author: [email protected] Abstract the traditional patterning processes. The QLED device achieved Photolithography is a high resolution and mature patterning maximum electroluminescence intensity of 23 770 cd/m2. [13] technique which has been widely used in semiconductor industry. For display application, a pixel consists of red (R), green (G) and In this work, we use photolithography to fine pattern the QD blue (B) side-by-side sub-pixels, which thereby requires a high layers. Because it is difficult to etch the QD layer, lift-off is used resolution patterning of the light-emission layers. -

Microfluidic Organs-On-Chips
PERSPECTIVE Microfluidic organs-on-chips Sangeeta N Bhatia1,2 & Donald E Ingber3–5 An organ-on-a-chip is a microfluidic cell culture device have limitations. For example, organoids are highly variable in size created with microchip manufacturing methods that contains and shape, and it is difficult to maintain cells in consistent positions continuously perfused chambers inhabited by living cells in these structures for extended analysis. Another drawback of 3D arranged to simulate tissue- and organ-level physiology. By models is that functional analysis of entrapped cells—for example, recapitulating the multicellular architectures, tissue-tissue to quantify transcellular transport, absorption or secretion—is often interfaces, physicochemical microenvironments and vascular hampered by the difficulty of sampling luminal contents, and it is perfusion of the body, these devices produce levels of tissue difficult to harvest cellular components for biochemical and genetic and organ functionality not possible with conventional 2D or analysis. In addition, many systems lack multiscale architecture and 3D culture systems. They also enable high-resolution, real- tissue-tissue interfaces, such as the interface between vascular endo- time imaging and in vitro analysis of biochemical, genetic and thelium and surrounding connective tissue and parenchymal cells, metabolic activities of living cells in a functional tissue and which are crucial to the function of nearly all organs. Furthermore, organ context. This technology has great potential to advance cells are usually not exposed to normal mechanical cues, including the study of tissue development, organ physiology and disease fluid shear stress, tension and compression, which influence organ etiology. In the context of drug discovery and development, development and function in health and disease8,9. -

Modification of the Microcontact Printing Process for Conductive Ink Printing Adam J
Worcester Polytechnic Institute Digital WPI Major Qualifying Projects (All Years) Major Qualifying Projects November 2017 Modification of the Microcontact Printing Process for Conductive Ink Printing Adam J. Cyran Worcester Polytechnic Institute Michaela Dowling Worcester Polytechnic Institute Follow this and additional works at: https://digitalcommons.wpi.edu/mqp-all Repository Citation Cyran, A. J., & Dowling, M. (2017). Modification of the Microcontact Printing Process for Conductive Ink Printing. Retrieved from https://digitalcommons.wpi.edu/mqp-all/2225 This Unrestricted is brought to you for free and open access by the Major Qualifying Projects at Digital WPI. It has been accepted for inclusion in Major Qualifying Projects (All Years) by an authorized administrator of Digital WPI. For more information, please contact [email protected]. Modification of the Microcontact Printing Process for Conductive Ink Printing A Major Qualifying Project Report submitted to the faculty of WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science NTB: Interstaatliche Hochschule fur Technik Buchs (The Interstate University of Applied Sciences of Technology Buchs) Project Advisor: Prof. Sarah Wodin-Schwartz, WPI Submitted By: Adam Cyran & Michaela Dowling November 3, 2017 Abstract -

Multifunctional Self-Assembled Monolayers Via Microcontact Printing and Degas-Driven Flow Guided Patterning
www.nature.com/scientificreports OPEN Multifunctional self-assembled monolayers via microcontact printing and degas-driven fow Received: 30 April 2018 Accepted: 27 October 2018 guided patterning Published: xx xx xxxx Sang Hun Lee1, Won-Yeop Rho2, Seon Joo Park3, Jinyeong Kim3, Oh Seok Kwon3 & Bong- Hyun Jun4 Soft lithography-based patterning techniques have been developed to investigate biological and chemical phenomena. Until now, micropatterning with various materials required multiple procedural steps such as repeating layer-by-layer patterning, aligning of stamps, and incubating printed inks. Herein, we describe a facile micropatterning method for producing chemically well-defned surface architectures by combining microcontact (µCP) and microfuidic vacuum-assisted degas-driven fow guided patterning (DFGP) with a poly(dimethylsiloxane) (PDMS) stamp. To demonstrate our concept, we fabricated a bi-composite micropatterned surface with diferent functional molecular inks such as fuorescein isothiocyanate labelled bovine serum albumin (FITC-BSA) and polyethylene glycol (PEG)- silane for a biomolecule array, and 3-aminopropyltriethoxysilane (APTES) and PEG-silane pattern for a self-assembled colloid gold nanoparticle monolayer. With a certain composition of molecular inks for the patterning, bi-composite surface patterns could be produced by this µCP-DFGP approach without any supplementary process. This patterning approach can be used in microfabrication and highly applicable to biomolecules and nanoparticles that spread as a monolayer. Micropatterns are now widely used in various research felds such as tissue engineering, developing bio-chips or sensors, etc1–4. Te creation of a micropattern with a complex architecture typically requires multiple processing steps, specialized facilities, and harsh conditions, including metal deposition and dry/wet etching5. -

Novel Microcontact Printing Technique for Multipatterning of Self-Assembled Monolayers
Sensors and Materials,, Vol.Vol. 17,17, No.No. 22 (2005)(2005) 049– 056 49 MYU Tokyo S & M 0585 Novel Microcontact Printing Technique for Multipatterning of Self-Assembled Monolayers Janggil Kim, Nobuyuki Takama and Beomjoon Kim* CIRMM, Institute of Industrial Science, University of Tokyo 4-6-1, Komaba, Meguro, Tokyo 153-0041, Japan (Received April 10, 2004; accepted November 16, 2004) Key words: self-assembled monolayer (SAM), microcontact printing (µCP), multilayer, laminated patterning, HDT, 11-MUA, DTS, bio-MEMS In this article, we focus on the development of a laminated patterning technique for various types of self-assembled monolayer (SAM) by microcontact printing (µCP). Using this technique, three types of functional SAM with hydrophobic and hydrophilic surface properties are patterned selectively on a silicon wafer. After the line shape of hexadecanethiol (CH3(CH2)15SH(CH3), HDT) is patterned on a Au substrate by µCP, the cross line pattern of 11-mercaptoundecanoic acid (HS(CH2)10COOH, 11-MUA) is transferred to the lami- nated Au substrate. Finally, the external area of the transferred SAM pattern on the sample is coated with dodecyltrichlorosilane (C12H25Cl3Si, DTS). The surface property of each SAM is measured by lateral force microscopy (LFM). This laminated patterning technique enables the fabrication of bio-micro-electro-mechanical system (bio-MEMS) devices by µCP. 1. Introduction Since it was introduced for the first time,(1) microcontact printing of a self-assembled monolayer (SAM) has gained great interest in micro- or nano-patterning technology as an alternative technique to conventional photolithography. Its high throughput and low cost by the master & replication system shows the possibility of mass production in practical industry. -
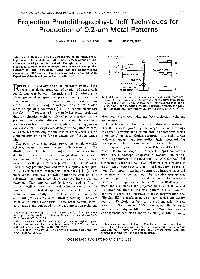
Projection Photolithography-Liftoff Techniques for Production of 0.2-Pm Metal Patterns
IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. ED-28, NO. 11, NOVEMBER 1981 1375 Projection Photolithography-Liftoff Techniques for Production of 0.2-pm Metal Patterns MARK D. FEUER AND DANIEL E. PROBER, MEMBER, IEEE GLASS FILTER Abstract-A technique whichallows the useof projection photo- \ yPHOTORESIST lithography with the photoresist liftoff process, for fabrication of sub- micrometer metal patterns, is described. Through-the-substrate (back- \II a projection) exposure of the photoresist produces the undercut profiles necessary forliftoff processing. Metal lines andsuperconducting microbridges of 0.2-pm width have been fabricated with this technique. I Experimental details and process limits are discussed. II OBJECTIVE IMMERSION OIL ECENT DEVELOPMENTS inmicrolithography have SUBSTRATE - (d) R made possible the production ofa variety of devices with PHOTORESIST %- I-l submicrometer (submicron) dimensions [ 11 , [2] , offering the (a) advantages of higherspeed and packing density.For many Fig. 1. Schematicdiagram of back-projectionand metal-liftoff pro- Josephson-effect devices in particular, submicron dimensions cedure. (a) Exposure system, employing a Zeiss optical microscope. Image of the mask is projected through the substrate, which is shown are essential for achieving optimalperformance over awide in sideview. (b) Schematic contours of constant exposure intensity. range of operatingconditions [3] . Forsubmicron pattern (c) After photoresist development and metallization. (d) After liftoff. transfer, liftoff processing [ 11 generally -

Stamps, Inks and Substrates: Polymers in Microcontact Printing
REVIEW www.rsc.org/polymers | Polymer Chemistry Stamps, inks and substrates: polymers in microcontact printing Tobias Kaufmann and Bart Jan Ravoo* Received 6th October 2009, Accepted 26th November 2009 First published as an Advance Article on the web 11th January 2010 DOI: 10.1039/b9py00281b Microcontact printing (mCP) is a straightforward method for the preparation of micro- and nanostructured surfaces. The key element in mCP is a polymeric stamp with a relief pattern. This stamp is ‘‘inked’’ and put in contact with the substrate surface. Ideally, the ink is transferred from stamp to substrate only in the area of contact. This review focuses on the important role of polymers in mCP. First of all, polymers are the material of choice to make mCP stamps. Furthermore, mCP is a useful method for preparing microstructured polymer surfaces. Polymers can be applied as inks in mCP so that microstructured polymer surfaces are obtained in a single printing step. Microstructured polymer surfaces can also be obtained by mCP on polymer substrates. A wide range of inks – including polymer inks – can be patterned on polymer substrates by mCP. In short, polymers are widely used as stamps, inks and substrates in mCP and we have organized this review accordingly. 1. Introduction substrate surface. Ideally, the ink is transferred from stamp to substrate only in the area of contact. The process of mCP is Microcontact printing (mCP) is a sophisticated version of schematically depicted in Fig. 1. a simple stamping process that is familiar even to most children. mCP was developed in the early 1990s by Kumar and White- Similar to conventional printing, mCP also involves an ink, sides for the patterned transfer of thiols onto Au surfaces by a substrate and a stamp. -

And Nanolithography Techniques and Their Applications
Review on Micro- and Nanolithography Techniques and their Applications Alongkorn Pimpin* and Werayut Srituravanich** Department of Mechanical Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand E-mail: [email protected]*, [email protected]** Abstract. This article reviews major micro- and nanolithography techniques and their applications from commercial micro devices to emerging applications in nanoscale science and engineering. Micro- and nanolithography has been the key technology in manufacturing of integrated circuits and microchips in the semiconductor industry. Such a technology is also sparking revolutionizing advancements in nanotechnology. The lithography techniques including photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography and scanning probe lithography are discussed. Furthermore, their applications are summarized into four major areas: electronics and microsystems, medical and biotech, optics and photonics, and environment and energy harvesting. Keywords: Nanolithography, photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography, scanning probe lithography, dip-pen lithography, microsystems, MEMS, nanoscience, nanotechnology, nano-engineering. ENGINEERING JOURNAL Volume 16 Issue 1 Received 18 August 2011 Accepted 8 November Published 1 January 2012 Online at http://www.engj.org DOI:10.4186/ej.2012.16.1.37 DOI:10.4186/ej.2012.16.1.37 1. Introduction For decades, micro- and nanolithography technology has been contributed to the manufacturing of integrated circuits (ICs) and microchips. This advance in the semiconductor and IC industry has led to a new paradigm of the information revolution via computers and the internet. Micro- and nanolithography is the technology that is used to create patterns with a feature size ranging from a few nanometers up to tens of millimeters. -

Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
micromachines Review Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems Jessica Ortigoza-Diaz 1, Kee Scholten 1 ID , Christopher Larson 1 ID , Angelica Cobo 1, Trevor Hudson 1, James Yoo 1 ID , Alex Baldwin 1 ID , Ahuva Weltman Hirschberg 1 and Ellis Meng 1,2,* 1 Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA; [email protected] (J.O.-D.); [email protected] (K.S.); [email protected] (C.L.); [email protected] (A.C.); [email protected] (T.H.); [email protected] (J.Y.); [email protected] (A.B.); [email protected] (A.W.H.) 2 Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA * Correspondence: [email protected]; Tel.: +1-213-740-6952 Received: 31 July 2018; Accepted: 18 August 2018; Published: 22 August 2018 Abstract: Parylene C is a promising material for constructing flexible, biocompatible and corrosion- resistant microelectromechanical systems (MEMS) devices. Historically, Parylene C has been employed as an encapsulation material for medical implants, such as stents and pacemakers, due to its strong barrier properties and biocompatibility. In the past few decades, the adaptation of planar microfabrication processes to thin film Parylene C has encouraged its use as an insulator, structural and substrate material for MEMS and other microelectronic devices. However, Parylene C presents unique challenges during microfabrication and during use with liquids, especially for flexible, thin film electronic devices. In particular, the flexibility and low thermal budget of Parylene C require modification of the fabrication techniques inherited from silicon MEMS, and poor adhesion at Parylene-Parylene and Parylene-metal interfaces causes device failure under prolonged use in wet environments. -

Fundimentals of Photolithography
FUNDIMENTALS OF PHOTOLITHOGRAPHY One of the most widely used methods for creating nanoscale circuit components is Photolithography. The word lithography is derived from the Greek words lithos (stone) and graphein (to write) and finds its roots in a process invented by Aloys Senefelder in 1796. By treating a piece of limestone with certain chemicals, Senefelder was then able to transfer an image carved into the stone onto a piece of paper. This was done by coating certain parts of the porous limestone with a water repellant substance. When ink was applied to the stone it would only adhere to the untreated hydrophilic areas, and hence the image carved into the rock could be transferred repeatedly onto paper. Senefelder's technique is still used in some artistry applications today. As time progressed and technology improved, lithography methods evolved. In the 1820's a French scientist by the name of Nicephore Niepce developed the first photoresist, a component fundamental to photolithography. A photoresist is a substance that undergoes a chemical reaction when it is eXposed to light. Niepce's photoresist was a material known as Bitumen of Judea, a kind of naturally occurring asphalt. A sheet of stone, metal, or glass was coated with a thin layer of this bitumen, which became less soluble where it was eXposed to light. Areas that were uneXposed could then be removed using a solvent, and the resultant exposed areas of the sheet were etched using a chemical bath. After the remaining photoresist was removed, the sheet could then be used as a printing plate. Photolithography today is in many ways similar to the original process invented by Niepce. -

Photolithography for the Investigation of Nanostructures
Photolithography for the Investigation of Nanostructures ---------------------------------------------------------- A Thesis Presented to the Honors Tutorial College, Ohio University ---------------------------------------------------------- In Partial Fulfillment of the Requirements for Graduation from the Honors Tutorial College with the degree of Bachelor of Science in Physics ---------------------------------------------------------- Helen M. Cothrel April 2015 This thesis has been approved by the Honors Tutorial College and the Department of Physics and Astronomy ________________________________________________ Dr. Eric Stinaff Professor, Physics and Astronomy Thesis adviser ________________________________________________ Dr. David Drabold Distinguished Professor, Physics and Astronomy Honors Tutorial College Director of Studies, Physics and Astronomy ________________________________________________ Dr. Jeremy Webster Dean, Honors Tutorial College 2 Table of Contents List of Figures ................................................................................................................. 4 Abstract ........................................................................................................................... 8 Introduction and Background ....................................................................................... 10 Photolithography ....................................................................................................... 11 Quantum Dots .......................................................................................................... -

Molecular Scale Imaging with a Smooth Superlens
Molecular Scale Imaging with a Smooth Superlens Pratik Chaturvedi1, Wei Wu2, VJ Logeeswaran3, Zhaoning Yu2, M. Saif Islam3, S.Y. Wang2, R. Stanley Williams2, & Nicholas Fang1* 1Department of Mechanical Science & Engineering, University of Illinois at Urbana- Champaign, 1206 W. Green St., Urbana, IL 61801, USA. 2Information & Quantum Systems Lab, Hewlett-Packard Laboratories, 1501 Page Mill Rd, MS 1123, Palo Alto, CA 94304, USA. 3Department of Electrical & Computer Engineering, Kemper Hall, University of California at Davis, One Shields Ave, Davis, CA 95616, USA. * Corresponding author Email: [email protected] RECEIVED DATE Abstract We demonstrate a smooth and low loss silver (Ag) optical superlens capable of resolving features at 1/12th of the illumination wavelength with high fidelity. This is made possible by utilizing state-of-the-art nanoimprint technology and intermediate 1 wetting layer of germanium (Ge) for the growth of flat silver films with surface roughness at sub-nanometer scales. Our measurement of the resolved lines of 30nm half-pitch shows a full-width at half-maximum better than 37nm, in excellent agreement with theoretical predictions. The development of this unique optical superlens lead promise to parallel imaging and nanofabrication in a single snapshot, a feat that are not yet available with other nanoscale imaging techniques such as atomic force microscope or scanning electron microscope. λ = 380nm 250nm The resolution of optical images has historically been constrained by the wavelength of light, a well known physical law which is termed as the diffraction limit. Conventional optical imaging is only capable of focusing the propagating components from the source. The evanescent components which carry the subwavelength information exponentially decay in a medium with positive permittivity (ε), and positive permeability (µ) and hence, are lost before making it to the image plane.