Anti-HIST3H2A Polyclonal Antibody (DPAB- DC3769) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
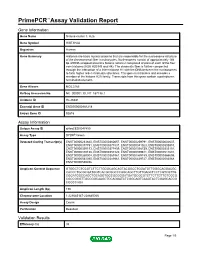
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name histone cluster 3, H2a Gene Symbol HIST3H2A Organism Human Gene Summary Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Nucleosomes consist of approximately 146 bp of DNA wrapped around a histone octamer composed of pairs of each of the four core histones (H2A H2B H3 and H4). The chromatin fiber is further compacted through the interaction of a linker histone H1 with the DNA between the nucleosomes to form higher order chromatin structures. This gene is intronless and encodes a member of the histone H2A family. Transcripts from this gene contain a palindromic termination element. Gene Aliases MGC3165 RefSeq Accession No. NC_000001.10, NT_167186.1 UniGene ID Hs.26331 Ensembl Gene ID ENSG00000181218 Entrez Gene ID 92815 Assay Information Unique Assay ID qHsaCED0047450 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000233840, ENST00000360657, ENST00000259791, ENST00000602637, ENST00000377791, ENST00000377831, ENST00000341023, ENST00000303910, ENST00000359193, ENST00000377459, ENST00000358739, ENST00000333151, ENST00000330180, ENST00000357320, ENST00000359611, ENST00000511601, ENST00000260008, ENST00000369161, ENST00000369159, ENST00000366695, ENST00000543095, ENST00000389961, ENST00000439727, ENST00000583968, ENST00000580456 Amplicon Context Sequence GTGGCTCTCCGTCTTCTTGGGCAGCAGTACGGCCTGGATGTTGGGCAGGACGC CACCCTGCGCGATGGTCACGCGGCCCAGCAGCTTGTTGAGCTCCTCGTCGTTG CGGATGGCCAGCTGCAGGTGGCGCGGGATGATGCGCGTCTTCTTGTTGTCGCG -

A Mutation in Histone H2B Represents a New Class of Oncogenic Driver
Author Manuscript Published OnlineFirst on July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. A Mutation in Histone H2B Represents A New Class Of Oncogenic Driver Richard L. Bennett1, Aditya Bele1, Eliza C. Small2, Christine M. Will2, Behnam Nabet3, Jon A. Oyer2, Xiaoxiao Huang1,9, Rajarshi P. Ghosh4, Adrian T. Grzybowski5, Tao Yu6, Qiao Zhang7, Alberto Riva8, Tanmay P. Lele7, George C. Schatz9, Neil L. Kelleher9 Alexander J. Ruthenburg5, Jan Liphardt4 and Jonathan D. Licht1 * 1 Division of Hematology/Oncology, University of Florida Health Cancer Center, Gainesville, FL 2 Division of Hematology/Oncology, Northwestern University 3 Department of Cancer Biology, Dana Farber Cancer Institute and Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School 4 Department of Bioengineering, Stanford University 5 Department of Molecular Genetics and Cell Biology, The University of Chicago 6 Department of Chemistry, Tennessee Technological University 7 Department of Chemical Engineering, University of Florida 8 Bioinformatics Core, Interdisciplinary Center for Biotechnology Research, University of Florida 9 Department of Chemistry, Northwestern University, Evanston IL 60208 Running title: Histone mutations in cancer *Corresponding Author: Jonathan D. Licht, MD The University of Florida Health Cancer Center Cancer and Genetics Research Complex, Suite 145 2033 Mowry Road Gainesville, FL 32610 352-273-8143 [email protected] Disclosures: The authors have no conflicts of interest to declare Downloaded from cancerdiscovery.aacrjournals.org on September 27, 2021. © 2019 American Association for Cancer Research. Author Manuscript Published OnlineFirst on July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -

A Discovery Resource of Rare Copy Number Variations in Individuals with Autism Spectrum Disorder
INVESTIGATION A Discovery Resource of Rare Copy Number Variations in Individuals with Autism Spectrum Disorder Aparna Prasad,* Daniele Merico,* Bhooma Thiruvahindrapuram,* John Wei,* Anath C. Lionel,*,† Daisuke Sato,* Jessica Rickaby,* Chao Lu,* Peter Szatmari,‡ Wendy Roberts,§ Bridget A. Fernandez,** Christian R. Marshall,*,†† Eli Hatchwell,‡‡ Peggy S. Eis,‡‡ and Stephen W. Scherer*,†,††,1 *The Centre for Applied Genomics, Program in Genetics and Genome Biology, The Hospital for Sick Children, Toronto M5G 1L7, Canada, †Department of Molecular Genetics, University of Toronto, Toronto M5G 1L7, Canada, ‡Offord Centre for Child Studies, Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton L8P 3B6, § Canada, Autism Research Unit, The Hospital for Sick Children, Toronto M5G 1X8, Canada, **Disciplines of Genetics and Medicine, Memorial University of Newfoundland, St. John’s, Newfoundland A1B 3V6, Canada, ††McLaughlin Centre, University of Toronto, Toronto M5G 1L7, Canada, and ‡‡Population Diagnostics, Inc., Melville, New York 11747 ABSTRACT The identification of rare inherited and de novo copy number variations (CNVs) in human KEYWORDS subjects has proven a productive approach to highlight risk genes for autism spectrum disorder (ASD). A rare variants variety of microarrays are available to detect CNVs, including single-nucleotide polymorphism (SNP) arrays gene copy and comparative genomic hybridization (CGH) arrays. Here, we examine a cohort of 696 unrelated ASD number cases using a high-resolution one-million feature CGH microarray, the majority of which were previously chromosomal genotyped with SNP arrays. Our objective was to discover new CNVs in ASD cases that were not detected abnormalities by SNP microarray analysis and to delineate novel ASD risk loci via combined analysis of CGH and SNP array cytogenetics data sets on the ASD cohort and CGH data on an additional 1000 control samples. -

A Mutation in Histone H2B Represents a New Class of Oncogenic Driver
Published OnlineFirst July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 RESEARCH ARTICLE A Mutation in Histone H2B Represents a New Class of Oncogenic Driver Richard L. Bennett1, Aditya Bele1, Eliza C. Small2, Christine M. Will2, Behnam Nabet3, Jon A. Oyer2, Xiaoxiao Huang1,4, Rajarshi P. Ghosh5, Adrian T. Grzybowski6, Tao Yu7, Qiao Zhang8, Alberto Riva9, Tanmay P. Lele8, George C. Schatz4, Neil L. Kelleher4, Alexander J. Ruthenburg6, Jan Liphardt5, and Jonathan D. Licht1 Downloaded from cancerdiscovery.aacrjournals.org on September 30, 2021. © 2019 American Association for Cancer Research. Published OnlineFirst July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 ABSTRACT By examination of the cancer genomics database, we identified a new set of mutations in core histones that frequently recur in cancer patient samples and are predicted to disrupt nucleosome stability. In support of this idea, we characterized a glutamate to lysine mutation of histone H2B at amino acid 76 (H2B-E76K), found particularly in bladder and head and neck cancers, that disrupts the interaction between H2B and H4. Although H2B-E76K forms dimers with H2A, it does not form stable histone octamers with H3 and H4 in vitro, and when recon- stituted with DNA forms unstable nucleosomes with increased sensitivity to nuclease. Expression of the equivalent H2B mutant in yeast restricted growth at high temperature and led to defective nucleosome-mediated gene repression. Significantly, H2B-E76K expression in the normal mammary epithelial cell line MCF10A increased cellular proliferation, cooperated with mutant PIK3CA to pro- mote colony formation, and caused a significant drift in gene expression and fundamental changes in chromatin accessibility, particularly at gene regulatory elements. -

Noncoding Rnas As Novel Pancreatic Cancer Targets
NONCODING RNAS AS NOVEL PANCREATIC CANCER TARGETS by Amy Makler A Thesis Submitted to the Faculty of The Charles E. Schmidt College of Science In Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, FL August 2018 Copyright 2018 by Amy Makler ii ACKNOWLEDGEMENTS I would first like to thank Dr. Narayanan for his continuous support, constant encouragement, and his gentle, but sometimes critical, guidance throughout the past two years of my master’s education. His faith in my abilities and his belief in my future success ensured I continue down this path of research. Working in Dr. Narayanan’s lab has truly been an unforgettable experience as well as a critical step in my future endeavors. I would also like to extend my gratitude to my committee members, Dr. Binninger and Dr. Jia, for their support and suggestions regarding my thesis. Their recommendations added a fresh perspective that enriched our initial hypothesis. They have been indispensable as members of my committee, and I thank them for their contributions. My parents have been integral to my successes in life and their support throughout my education has been crucial. They taught me to push through difficulties and encouraged me to pursue my interests. Thank you, mom and dad! I would like to thank my boyfriend, Joshua Disatham, for his assistance in ensuring my writing maintained a logical progression and flow as well as his unwavering support. He was my rock when the stress grew unbearable and his encouraging words kept me pushing along. -

The Human Canonical Core Histone Catalogue David Miguel Susano Pinto*, Andrew Flaus*,†
bioRxiv preprint doi: https://doi.org/10.1101/720235; this version posted July 30, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The Human Canonical Core Histone Catalogue David Miguel Susano Pinto*, Andrew Flaus*,† Abstract Core histone proteins H2A, H2B, H3, and H4 are encoded by a large family of genes dis- tributed across the human genome. Canonical core histones contribute the majority of proteins to bulk chromatin packaging, and are encoded in 4 clusters by 65 coding genes comprising 17 for H2A, 18 for H2B, 15 for H3, and 15 for H4, along with at least 17 total pseudogenes. The canonical core histone genes display coding variation that gives rise to 11 H2A, 15 H2B, 4 H3, and 2 H4 unique protein isoforms. Although histone proteins are highly conserved overall, these isoforms represent a surprising and seldom recognised variation with amino acid identity as low as 77 % between canonical histone proteins of the same type. The gene sequence and protein isoform diversity also exceeds com- monly used subtype designations such as H2A.1 and H3.1, and exists in parallel with the well-known specialisation of variant histone proteins. RNA sequencing of histone transcripts shows evidence for differential expression of histone genes but the functional significance of this variation has not yet been investigated. To assist understanding of the implications of histone gene and protein diversity we have catalogued the entire human canonical core histone gene and protein complement. -

PDF Download
Histone H2A (Phospho Ser129) Polyclonal Antibody Catalog No : YM3277 Reactivity : Human,Mouse,Rat Applications : WB Gene Name : HIST1H2AG/HIST1H2AI/HIST1H2AK/HIST1H2AL/HIST1H2AM/HIST2H2AA3 /HIST2H2AA4/HIST3H2A Protein Name : Histone H2A type 1/Histone H2A type 2/Histone H2A type 3 Human Gene Id : 8329/8330/8332/8336/8969/723790/8337/92815 Human Swiss Prot P0C0S8/Q6FI13/Q7L7L0 No : Mouse Gene Id : 319164/15267/319162 Rat Gene Id : 365877/64646 Rat Swiss Prot No : P02262/P0CC09/Q4FZT6 Immunogen : Synthetic Peptide of Histone H2A (Phospho Ser129) Specificity : The antibody detects endogenous Histone H2A (Phospho Ser129) protein. Formulation : PBS, pH 7.4, containing 0.5%BSA, 0.02% sodium azide as Preservative and 50% Glycerol. Source : Rabbit Dilution : WB: 1:1000-2000 Purification : The antibody was affinity-purified from rabbit antiserum by affinity- chromatography using specific immunogen. Storage Stability : -20°C/1 year Molecularweight : 14091/14095/14121 1 / 3 Observed Band : 14 Cell Pathway : Systemic lupus erythematosus, Background : histone cluster 1 H2A family member i(HIST1H2AI) Homo sapiens Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Two molecules of each of the four core histones (H2A, H2B, H3, and H4) form an octamer, around which approximately 146 bp of DNA is wrapped in repeating units, called nucleosomes. The linker histone, H1, interacts with linker DNA between nucleosomes and functions in the compaction of chromatin into higher order structures. This gene is intronless and encodes a replication-dependent histone that is a member of the histone H2A family. Transcripts from this gene lack polyA tails but instead contain a palindromic termination element. -

Fine-Mapping Quantitative Trait Loci Affecting Murine External Ear Tissue Regeneration in the LG/J by SM/J Advanced Intercross Line
Heredity (2014) 112, 508–518 & 2014 Macmillan Publishers Limited All rights reserved 0018-067X/14 www.nature.com/hdy ORIGINAL ARTICLE Fine-mapping quantitative trait loci affecting murine external ear tissue regeneration in the LG/J by SM/J advanced intercross line JM Cheverud1,4, HA Lawson1,5, K Bouckaert1, AV Kossenkov2, LC Showe2, L Cort3, EP Blankenhorn3, K Bedelbaeva2, D Gourevitch2, Y Zhang2 and E Heber-Katz2 External ear hole closure in LG/J mice represents a model of regenerative response. It is accompanied by the formation of a blastema-like structure and the re-growth of multiple tissues, including cartilage. The ability to regenerate tissue is heritable. An F34 advanced intercross line of mice (Wustl:LG,SM-G34) was generated to identify genomic loci involved in ear hole closure over a 30-day healing period. We mapped 19 quantitative trait loci (QTL) for ear hole closure. Individual gene effects are relatively small (0.08 mm), and most loci have co-dominant effects with phenotypically intermediate heterozygotes. QTL support regions were limited to a median size of 2 Mb containing a median of 19 genes. Positional candidate genes were evaluated using differential transcript expression between LG/J and SM/J healing tissue, function analysis and bioinformatic analysis of single-nucleotide polymorphisms in and around positional candidate genes of interest. Analysis of the set of 34 positional candidate genes and those displaying expression differences revealed over-representation of genes involved in cell cycle regulation/DNA damage, cell migration and adhesion, developmentally related genes and metabolism. This indicates that the healing phenotype in LG/J mice involves multiple physiological mechanisms. -

BMC Genetics Biomed Central
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector BMC Genetics BioMed Central Research article Open Access A novel replication-independent histone H2a gene in mouse Hiromi Nishida*, Takahiro Suzuki, Yasuhiro Tomaru and Yoshihide Hayashizaki Address: Laboratory for Genome Exploration Research Group, RIKEN Genomic Sciences Center (GSC), RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan Email: Hiromi Nishida* - [email protected]; Takahiro Suzuki - [email protected]; Yasuhiro Tomaru - [email protected]; Yoshihide Hayashizaki - [email protected] * Corresponding author Published: 19 February 2005 Received: 07 October 2004 Accepted: 19 February 2005 BMC Genetics 2005, 6:10 doi:10.1186/1471-2156-6-10 This article is available from: http://www.biomedcentral.com/1471-2156/6/10 © 2005 Nishida et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: An uncharacterized histone H2a-coding transcript (E130307C13) has been cloned from a mouse full-length cDNA library. This transcript is encoded on chromosome 6, approximately 4 kb upstream of a histone H4 gene, Hist4h4. The proteins encoded by this transcript and the human H2afj mRNA isoform-2 have the highest amino acid similarity. In this paper, we characterize it from the expression pattern given by quantitative RT-PCR. Results: Quantitative RT-PCR indicated that the gene that encodes E130307C13 (E130307C13) is regulated in a replication-independent manner, and therefore it is H2afj. -

HIST3H2A Is Differentially Expressed in Brain Metastatic Breast Cancer
1 HIST3H2A is differentially expressed in the brain metastases of patients with metastatic breast cancer. 2 Shahan Mamoor, MS1 3 [email protected] 4 East Islip, NY USA 5 6 Metastasis to the brain is a clinical problem in patients with breast cancer1-3. We mined published microarray data4,5 to compare primary and metastatic tumor transcriptomes to discover genes associated 7 with brain metastasis in patients with metastatic breast cancer. We found that histone cluster 3, H2a, encoded by HIST3H2A, was among the genes whose expression was most different in the brain 8 metastases of patients with metastatic breast cancer as compared to normal breast tissues and primary 9 tumors of the breast. HIST3H2A mRNA was present at increased quantities in brain metastatic tissues as compared to primary tumors of the breast and to normal breast tissues. Up-regulation of HIST3H2A 10 expression may be relevant to the biology by which tumor cells metastasize from the breast to the brain in humans with metastatic breast cancer. 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Keywords: breast cancer, metastasis, brain metastases, central nervous system metastases, histone cluster 3, H2a, HIST3H2A, systems biology of breast cancer, targeted therapeutics in breast cancer. 28 PAGE 1 1 One report described a 34% incidence of central nervous system metastases in patients treated with trastuzumab for breast cancer2. More recently, the NEfERT-T clinical trial6 which compared 2 administration of either neratinib or trastuzumab in conjunction with paclitaxel demonstrated that in a randomized, controlled setting, in breast cancer patients treated with neratinib, not only was the incidence 3 of central nervous system recurrence significantly lower, the time to central nervous system metastasis 4 was significantly delayed as compared to patients administered trastuzumab. -

1471-2164-6-108.Pdf
BMC Genomics BioMed Central Research article Open Access Comparative analysis of expression of histone H2a genes in mouse Hiromi Nishida*, Takahiro Suzuki, Hiroki Ookawa, Yasuhiro Tomaru and Yoshihide Hayashizaki Address: Laboratory for Genome Exploration Research Group, RIKEN Genomic Sciences Center (GSC), RIKEN Yokohama Institute, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan Email: Hiromi Nishida* - [email protected]; Takahiro Suzuki - [email protected]; Hiroki Ookawa - [email protected]; Yasuhiro Tomaru - [email protected]; Yoshihide Hayashizaki - [email protected] * Corresponding author Published: 13 August 2005 Received: 23 February 2005 Accepted: 13 August 2005 BMC Genomics 2005, 6:108 doi:10.1186/1471-2164-6-108 This article is available from: http://www.biomedcentral.com/1471-2164/6/108 © 2005 Nishida et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: At least 18 replication-dependent histone H2a genes are distributed in 3 Hist gene clusters on different chromosomes of the mouse genome. In this analysis we designed specific PCR primers for each histone H2a transcript and studied the expression levels and patterns using quantitative RT-PCR (qRT-PCR). In addition, we compared histone H3 K9 acetylation levels in the promoter regions of H2a genes by ChIP (chromatin immunoprecipitation) – quantitative PCR (qPCR) analysis. Results: RT-PCR analysis indicated that all 20 histone H2a genes assessed in this study are expressed. -

Human Recombinant Protein – TP710343
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TP710343 H2AW (NM_033445) Human Recombinant Protein Product data: Product Type: Recombinant Proteins Description: Purified recombinant protein of Human histone cluster 3, H2a (HIST3H2A), full length, with with C-terminal DDK tag, expressed in sf9, 20ug Species: Human Expression Host: Sf9 Tag: C-DDK Predicted MW: 13.9 kDa Concentration: >50 ug/mL as determined by microplate BCA method Purity: > 80% as determined by SDS-PAGE and Coomassie blue staining Buffer: 50mM Tris-HCl, pH8.0, 100mM glycine, 10% glycerol Storage: Store at -80°C. Stability: Stable for 12 months from the date of receipt of the product under proper storage and handling conditions. Avoid repeated freeze-thaw cycles. RefSeq: NP_254280 Locus ID: 92815 UniProt ID: Q7L7L0 RefSeq Size: 496 Cytogenetics: 1q42.13 RefSeq ORF: 390 Synonyms: HIST3H2A Summary: Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Nucleosomes consist of approximately 146 bp of DNA wrapped around a histone octamer composed of pairs of each of the four core histones (H2A, H2B, H3, and H4). The chromatin fiber is further compacted through the interaction of a linker histone, H1, with the DNA between the nucleosomes to form higher order chromatin structures. This gene is intronless and encodes a replication-dependent histone that is a member of the histone H2A family. Transcripts from this gene contain a palindromic termination element.