Thin-Sections of Marine Bivalve Shells: a Window to Environmental Reconstruction on a Daily Scale?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bankia Setacea Class: Bivalvia, Heterodonta, Euheterodonta
Phylum: Mollusca Bankia setacea Class: Bivalvia, Heterodonta, Euheterodonta Order: Imparidentia, Myida The northwest or feathery shipworm Family: Pholadoidea, Teredinidae, Bankiinae Taxonomy: The original binomen for Bankia the presence of long siphons. Members of setacea was Xylotrya setacea, described by the family Teredinidae are modified for and Tryon in 1863 (Turner 1966). William Leach distiguished by a wood-boring mode of life described several molluscan genera, includ- (Sipe et al. 2000), pallets at the siphon tips ing Xylotrya, but how his descriptions were (see Plate 394C, Coan and Valentich-Scott interpreted varied. Although Menke be- 2007) and distinct anterior shell indentation. lieved Xylotrya to be a member of the Phola- They are commonly called shipworms (though didae, Gray understood it as a member of they are not worms at all!) and bore into many the Terdinidae and synonyimized it with the wooden structures. The common name ship- genus Bankia, a genus designated by the worm is based on their vermiform morphology latter author in 1842. Most authors refer to and a shell that only covers the anterior body Bankia setacea (e.g. Kozloff 1993; Sipe et (Ricketts and Calvin 1952; see images in al. 2000; Coan and Valentich-Scott 2007; Turner 1966). Betcher et al. 2012; Borges et al. 2012; Da- Body: Bizarrely modified bivalve with re- vidson and de Rivera 2012), although one duced, sub-globular body. For internal anato- recent paper sites Xylotrya setacea (Siddall my, see Fig. 1, Canadian…; Fig. 1 Betcher et et al. 2009). Two additional known syno- al. 2012. nyms exist currently, including Bankia Color: osumiensis, B. -

The Effects of Environment on Arctica Islandica Shell Formation and Architecture
Biogeosciences, 14, 1577–1591, 2017 www.biogeosciences.net/14/1577/2017/ doi:10.5194/bg-14-1577-2017 © Author(s) 2017. CC Attribution 3.0 License. The effects of environment on Arctica islandica shell formation and architecture Stefania Milano1, Gernot Nehrke2, Alan D. Wanamaker Jr.3, Irene Ballesta-Artero4,5, Thomas Brey2, and Bernd R. Schöne1 1Institute of Geosciences, University of Mainz, Joh.-J.-Becherweg 21, 55128 Mainz, Germany 2Alfred Wegener Institute for Polar and Marine Research, Am Handelshafen 12, 27570 Bremerhaven, Germany 3Department of Geological and Atmospheric Sciences, Iowa State University, Ames, Iowa 50011-3212, USA 4Royal Netherlands Institute for Sea Research and Utrecht University, P.O. Box 59, 1790 AB Den Burg, Texel, the Netherlands 5Department of Animal Ecology, VU University Amsterdam, Amsterdam, the Netherlands Correspondence to: Stefania Milano ([email protected]) Received: 27 October 2016 – Discussion started: 7 December 2016 Revised: 1 March 2017 – Accepted: 4 March 2017 – Published: 27 March 2017 Abstract. Mollusks record valuable information in their hard tribution, and (2) scanning electron microscopy (SEM) was parts that reflect ambient environmental conditions. For this used to detect changes in microstructural organization. Our reason, shells can serve as excellent archives to reconstruct results indicate that A. islandica microstructure is not sen- past climate and environmental variability. However, animal sitive to changes in the food source and, likely, shell pig- physiology and biomineralization, which are often poorly un- ment are not altered by diet. However, seawater temperature derstood, can make the decoding of environmental signals had a statistically significant effect on the orientation of the a challenging task. -

Siliqua Patula Class: Bivalvia; Heterodonta Order: Veneroida the Flat Razor Clam Family: Pharidae
Phylum: Mollusca Siliqua patula Class: Bivalvia; Heterodonta Order: Veneroida The flat razor clam Family: Pharidae Taxonomy: The familial designation of this (see Plate 397G, Coan and Valentich-Scott species has changed frequently over time. 2007). Previously in the Solenidae, current intertidal Body: (see Plate 29 Ricketts and Calvin guides include S. patula in the Pharidae (e.g., 1952; Fig 259 Kozloff 1993). Coan and Valentich-Scott 2007). The superfamily Solenacea includes infaunal soft Color: bottom dwelling bivalves and contains the two Interior: (see Fig 5, Pohlo 1963). families: Solenidae and Pharidae (= Exterior: Cultellidae, von Cosel 1993) (Remacha- Byssus: Trivino and Anadon 2006). In 1788, Dixon Gills: described S. patula from specimens collected Shell: The shell in S. patula is thin and with in Alaska (see Range) and Conrad described sharp (i.e., razor-like) edges and a thin profile the same species, under the name Solen (Fig. 4). Thin, long, fragile shell (Ricketts and nuttallii from specimens collected in the Calvin 1952), with gapes at both ends Columbia River in 1838 (Weymouth et al. (Haderlie and Abbott 1980). Shell smooth 1926). These names were later inside and out (Dixon 1789), elongate, rather synonymized, thus known synonyms for cylindrical and the length is about 2.5 times Siliqua patula include Solen nuttallii, the width. Solecurtus nuttallii. Occasionally, researchers Interior: Prominent internal vertical also indicate a subspecific epithet (e.g., rib extending from beak to margin (Haderlie Siliqua siliqua patula) or variations (e.g., and Abbott 1980). Siliqua patula var. nuttallii, based on rib Exterior: Both valves are similar and morphology, see Possible gape at both ends. -

Softshell Clam
REFEqENCE COPY Do Not Remove from the Library 11 S. Fish and Wildlife Service Biological Report 82 (11.53) NationalWetlands Research Center TR EL-82-4 June 1986 700 Cajun Dome Boulevard Lafcryette, Louisiana 70506 Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (North Atlantic) SOFTSHELL CLAM Coastal Ecology Group Fish and Wildlife Service Waterways ~xperimentStation U.S. Department of the Interior U.S. Army Corps of Engineers Biological Report 82(11.53) TR EL-82-4 June 1986 Species Profiles: Life Histories and Environmental Requirements of Coastal Fish and Invertebrates (North Atlantic) SOFTSHELL CLAM Carter R. Newel1 Maine She1 lfish Research and Development Damariscotta, ME 04543 and Herbert Hidu Department of Ani ma1 and Veterinary Sciences University of Maine Orono, ME 04469 Project Officer John Parsons National Coastal Ecosystems Team U.S. Fish and Wildlife Service 1010 Gause Boulevard Slidell, LA 70458 Performed for Coastal Ecology Group Waterways Experiment Station U.S. Army Corps of Engineers Vicksburg, MS 39180 and National .Coastal Ecosystems Team Research and Development Fish and Wildlife Service U.S. Department of the Interior Washington, DC 20240 This series should be referenced as follows: U.S. Fish and Wildlife Service. 1983-19 . Species profiles: life histories and environmental requirements of coaxal fishes and invertebrates. U. S. Fish Wildl. Serv. Biol. Rep. 82(11). U. S. Army Corps of Engineers, TR EL-82-4. This profile should be cited as follows: Newell, C.R., and H. Hidu. 1986. Species profiles: life histories and envi ronmental requi rements of coastal fishes and invertebrates (North Atlantic) -- softshell clam. -

Biodiversità Ed Evoluzione
Allma Mater Studiiorum – Uniiversiità dii Bollogna DOTTORATO DI RICERCA IN BIODIVERSITÀ ED EVOLUZIONE Ciclo XXIII Settore/i scientifico-disciplinare/i di afferenza: BIO - 05 A MOLECULAR PHYLOGENY OF BIVALVE MOLLUSKS: ANCIENT RADIATIONS AND DIVERGENCES AS REVEALED BY MITOCHONDRIAL GENES Presentata da: Dr Federico Plazzi Coordinatore Dottorato Relatore Prof. Barbara Mantovani Dr Marco Passamonti Esame finale anno 2011 of all marine animals, the bivalve molluscs are the most perfectly adapted for life within soft substrata of sand and mud. Sir Charles Maurice Yonge INDEX p. 1..... FOREWORD p. 2..... Plan of the Thesis p. 3..... CHAPTER 1 – INTRODUCTION p. 3..... 1.1. BIVALVE MOLLUSKS: ZOOLOGY, PHYLOGENY, AND BEYOND p. 3..... The phylum Mollusca p. 4..... A survey of class Bivalvia p. 7..... The Opponobranchia: true ctenidia for a truly vexed issue p. 9..... The Autobranchia: between tenets and question marks p. 13..... Doubly Uniparental Inheritance p. 13..... The choice of the “right” molecular marker in bivalve phylogenetics p. 17..... 1.2. MOLECULAR EVOLUTION MODELS, MULTIGENE BAYESIAN ANALYSIS, AND PARTITION CHOICE p. 23..... CHAPTER 2 – TOWARDS A MOLECULAR PHYLOGENY OF MOLLUSKS: BIVALVES’ EARLY EVOLUTION AS REVEALED BY MITOCHONDRIAL GENES. p. 23..... 2.1. INTRODUCTION p. 28..... 2.2. MATERIALS AND METHODS p. 28..... Specimens’ collection and DNA extraction p. 30..... PCR amplification, cloning, and sequencing p. 30..... Sequence alignment p. 32..... Phylogenetic analyses p. 37..... Taxon sampling p. 39..... Dating p. 43..... 2.3. RESULTS p. 43..... Obtained sequences i p. 44..... Sequence analyses p. 45..... Taxon sampling p. 45..... Maximum Likelihood p. 47..... Bayesian Analyses p. 50..... Dating the tree p. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
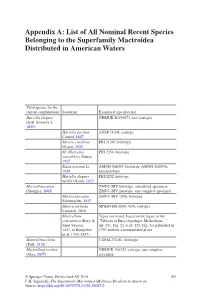
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

A Phylogenetic Backbone for Bivalvia: an RNA-Seq Approach
A phylogenetic backbone for Bivalvia: an RNA-seq approach The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation González, Vanessa L., Sónia C. S. Andrade, Rüdiger Bieler, Timothy M. Collins, Casey W. Dunn, Paula M. Mikkelsen, John D. Taylor, and Gonzalo Giribet. 2015. “A phylogenetic backbone for Bivalvia: an RNA-seq approach.” Proceedings of the Royal Society B: Biological Sciences 282 (1801): 20142332. doi:10.1098/rspb.2014.2332. http:// dx.doi.org/10.1098/rspb.2014.2332. Published Version doi:10.1098/rspb.2014.2332 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:14065405 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA A phylogenetic backbone for Bivalvia: an rspb.royalsocietypublishing.org RNA-seq approach Vanessa L. Gonza´lez1,†,So´nia C. S. Andrade1,‡,Ru¨diger Bieler2, Timothy M. Collins3, Casey W. Dunn4, Paula M. Mikkelsen5, Research John D. Taylor6 and Gonzalo Giribet1 1 Cite this article: Gonza´lez VL, Andrade SCS, Museum of Comparative Zoology, Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA Bieler R, Collins TM, Dunn CW, Mikkelsen PM, 2Integrative Research Center, Field Museum of Natural History, Chicago, IL 60605, USA Taylor JD, Giribet G. 2015 A phylogenetic 3Department of Biological Sciences, Florida International University, Miami, FL 33199, USA backbone for Bivalvia: an RNA-seq approach. -

Akut76004.Pdf
Institute of Marine Science University of Alaska Fairbanks, Alaska 99701 CLAN, MUSSEL, AND OYSTER RESOURCES OF ALASKA by A. J. Paul and Howard M. Feder NATIONALgg~C".~I'! t DEPOSITOR PELLLIBIiARY HUILQI'tlG URI,NAP,RAGAH4'.i l Bkf CAMPIJS NARRAGANSGT,Ri 02S82 IMS Report No. 76-4 D. W. Hood Sea Grant Report No. 76-6 Director April 1976 TABLE OF CONTENTS Preface Acknowledgement Sunnnary INTRODUCTION Factors Affecting Clam Densities Governmental Regulations Demand for Clara Products RAZORCLAM Siliqua pa&la! BUTTERCLAM Sazidomus gipantea! 16 BASKET COCKLE CLinocardium nuttal.lii! 19 LITTI.ENEGK cLAM Prot0thaca staminea! 21 SOFT-SHELLCLAM Ãpa az'encomia! 25 Hpa priapus! 28 THE TRUNCATE SOFT-SHELL Ãya tmncata! 29 PINKNECKCLAM +isu2a polymyma! 29 BUTTERFLY TELLIN Tsarina lactea! 32 ADDITIONAL CLAM SPECIES 32 BLUE MUSSEL Pfytilus eduHs! 33 OYSTERS 34 LITERATURE CITED 37 APPENDIX I. Classification of common Alaskan bivalves discussed in this report 40 APPENDIX I I Metric conversion values PREFACE This report is a compilation of data gathered in the course of a University of Alaska Sea Grant project, The BioZogg of FconomicaZLy Important BivaZves and Other MoZZuscs. This project concentrated on the study of hard shell clams and was designed to complement on-going Alaska Department of Fish and Game razor clam research. The primary purpose of this report is to provide the public with existing biological information on the clam, mussel, and oyster resources of the state. lt is intended to be supplementary to a previous report The AZaska CZarnFishery: A sue'ver and anaZgsis of economic potentcaZ, lMS Report No. R75-3, Sea Grant No. -

Mya Arenaria Class: Bivalvia; Heterodonta Order: Myoida Soft-Shelled Clam Family: Myidae
Phylum: Mollusca Mya arenaria Class: Bivalvia; Heterodonta Order: Myoida Soft-shelled clam Family: Myidae Taxonomy: Mya arenaria is this species cilia allow the style to rotate and press against original name and is almost exclusively used a gastric shield within the stomach, aiding in currently. However, the taxonomic history of digestion (Lawry 1987). In M. arenaria, the this species includes many synonyms, crystalline style can be regenerated after 74 overlapping descriptions, and/or subspecies days (Haderlie and Abbott 1980) and may (e.g. Mya hemphilli, Mya arenomya arenaria, contribute to the clam’s ability to live without Winckworth 1930; Bernard 1979). The oxygen for extended periods of time (Ricketts subgenera of Mya (Mya mya, Mya arenomya) and Calvin 1952). The ligament is white, were based on the presence or absence of a strong, and entirely internal (Kozloff 1993). subumbonal groove on the left valve and the Two types of gland cells (bacillary and goblet) morphology of the pallial sinus and pallial line comprise the pedal aperture gland or (see Bernard 1979). glandular cushion located within the pedal gape. It is situated adjacent to each of the Description two mantle margins and aids in the formation Size: Individuals range in size from 2–150 of pseudofeces from burrow sediments; the mm (Jacobson et al. 1975; Haderlie and structure of these glands may be of Abbott 1980; Kozloff 1993; Maximovich and phylogenetic relevance (Norenburg and Guerassimova 2003) and are, on average, Ferraris 1992). 50–100 mm (Fig. 1). Mean weight and length Exterior: were 74 grams and 8 cm (respectively) in Byssus: Wexford, Ireland (Cross et al. -

Population Dynamics of the Argentinean Surf Clams Donax Hanleyanus and Mesodesma Mactroides from Open-Atlantic Beaches Off Argentina
Population dynamics of the Argentinean surf clams Donax hanleyanus and Mesodesma mactroides from open-Atlantic beaches off Argentina Populationsdynamik der Argentinischen Brandungsmuscheln Donax hanleyanus und Mesodesma mactroides offener Atlantikstrände vor Argentinien Marko Herrmann Dedicated to my family Marko Herrmann Alfred Wegener Institute for Polar and Marine Research (AWI) Section of Marine Animal Ecology P.O. Box 120161 D-27515 Bremerhaven (Germany) [email protected] Submitted for the degree Dr. rer. nat. of the Faculty 2 of Biology and Chemistry University of Bremen (Germany), October 2008 Reviewer and principal supervisor: Prof. Dr. Wolf E. Arntz 1 Reviewer and co-supervisor: Dr. Jürgen Laudien 1 External Reviewer: Dr. Pablo E. Penchaszadeh 2 1 Alfred Wegener Institute for Polar and Marine Research (AWI) Section of Marine Animal Ecology P.O. Box 120161 D-27515 Bremerhaven (Germany) 2 Director of the Ecology Section at the Museo Argentino de Ciencias Naturales (MACN) - Bernardino Rivadavia Av. Angel Gallardo 470, 3° piso lab. 80 C1405DJR Buenos Aires (Argentina) Contents 1 Summary .................................................................................................... 5 1.1 English Version ........................................................................................... 5 1.2 Deutsche Version ........................................................................................ 9 1.3 Versión Español ........................................................................................ 13 2 Introduction -

Fossil Bivalves and the Sclerochronological Reawakening
Paleobiology, 2021, pp. 1–23 DOI: 10.1017/pab.2021.16 Review Fossil bivalves and the sclerochronological reawakening David K. Moss* , Linda C. Ivany, and Douglas S. Jones Abstract.—The field of sclerochronology has long been known to paleobiologists. Yet, despite the central role of growth rate, age, and body size in questions related to macroevolution and evolutionary ecology, these types of studies and the data they produce have received only episodic attention from paleobiologists since the field’s inception in the 1960s. It is time to reconsider their potential. Not only can sclerochrono- logical data help to address long-standing questions in paleobiology, but they can also bring to light new questions that would otherwise have been impossible to address. For example, growth rate and life-span data, the very data afforded by chronological growth increments, are essential to answer questions related not only to heterochrony and hence evolutionary mechanisms, but also to body size and organism ener- getics across the Phanerozoic. While numerous fossil organisms have accretionary skeletons, bivalves offer perhaps one of the most tangible and intriguing pathways forward, because they exhibit clear, typically annual, growth increments and they include some of the longest-lived, non-colonial animals on the planet. In addition to their longevity, modern bivalves also show a latitudinal gradient of increasing life span and decreasing growth rate with latitude that might be related to the latitudinal diversity gradient. Is this a recently developed phenomenon or has it characterized much of the group’s history? When and how did extreme longevity evolve in the Bivalvia? What insights can the growth increments of fossil bivalves provide about hypotheses for energetics through time? In spite of the relative ease with which the tools of sclerochronology can be applied to these questions, paleobiologists have been slow to adopt sclerochrono- logical approaches.