Origin, Evolution and Diversification of the Scandent Habit in Flowering Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

System Klasyfikacji Organizmów
1 SYSTEM KLASYFIKACJI ORGANIZMÓW Nie ma dziś ogólnie przyjętego systemu klasyfikacji organizmów. Nie udaje się osiągnąć zgodności nie tylko w odniesieniu do zakresów i rang poszczególnych taksonów, ale nawet co do zasad metodologicznych, na których klasyfikacja powinna być oparta. Zespołowe dzieła przeglądowe z reguły prezentują odmienne i wzajemnie sprzeczne podejścia poszczególnych autorów, bez nadziei na consensus. Nie ma więc mowy o skompilowaniu jednolitego schematu klasyfikacji z literatury i system przedstawiony poniżej nie daje nadziei na zaakceptowanie przez kogokolwiek poza kompilatorem. Przygotowany został w oparciu o kilka zasad (skądinąd bardzo kontrowersyjnych): (1) Identyfikowanie ga- tunków i ich klasyfikowanie w jednostki rodzajowe, rodzinowe czy rzędy jest zadaniem specjalistów i bez szcze- gółowych samodzielnych studiów nie można kwestionować wyników takich badań. (2) Podział świata żywego na królestwa, typy, gromady i rzędy jest natomiast domeną ewolucjonistów i dydaktyków. Powody, które posłużyły do wydzielenia jednostek powinny być jasno przedstawialne i zrozumiałe również dla niespecjalistów, albowiem (3) podstawowym zadaniem systematyki jest ułatwianie laikom i początkującym badaczom poruszanie się w obezwładniającej złożoności świata żywego. Wątpliwe jednak, by wystarczyło to do stworzenia zadowalającej klasyfikacji. W takiej sytuacji można jedynie przypomnieć, że lepszy ułomny system, niż żaden. Królestwo PROKARYOTA Chatton, 1938 DNA wyłącznie w postaci kolistej (genoforów), transkrypcja nie rozdzielona przestrzennie od translacji – rybosomy w tym samym przedzia- le komórki, co DNA. Oddział CYANOPHYTA Smith, 1938 (Myxophyta Cohn, 1875, Cyanobacteria Stanier, 1973) Stosunkowo duże komórki, dwuwarstwowa błona (Gram-ujemne), wewnętrzna warstwa mureinowa. Klasa CYANOPHYCEAE Sachs, 1874 Rząd Stigonematales Geitler, 1925; zigen – dziś Chlorofil a na pojedynczych tylakoidach. Nitkowate, rozgałęziające się, cytoplazmatyczne połączenia mię- Rząd Chroococcales Wettstein, 1924; 2,1 Ga – dziś dzy komórkami, miewają heterocysty. -

Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga
Subject BOTANY Paper No V Paper Code BOT521 Topic Taxonomy and Diversity of Seed Plant: Gymnosperms & Angiosperms Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants UNIT- I BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants Classification of Gymnosperms. # Robert Brown (1827) for the first time recognized Gymnosperm as a group distinct from angiosperm due to the presence of naked ovules. BENTHAM and HOOKSER (1862-1883) consider them equivalent to dicotyledons and monocotyledons and placed between these two groups of angiosperm. They recognized three classes of gymnosperm, Cyacadaceae, coniferac and gnetaceae. Later ENGLER (1889) created a group Gnikgoales to accommodate the genus giankgo. Van Tieghem (1898) treated Gymnosperm as one of the two subdivision of spermatophyte. To accommodate the fossil members three more classes- Pteridospermae, Cordaitales, and Bennettitales where created. Coulter and chamberlain (1919), Engler and Prantl (1926), Rendle (1926) and other considered Gymnosperm as a division of spermatophyta, Phanerogamia or Embryoptyta and they further divided them into seven orders: - i) Cycadofilicales ii) Cycadales iii) Bennettitales iv) Ginkgoales v) Coniferales vi) Corditales vii) Gnetales On the basis of wood structure steward (1919) divided Gymnosperm into two classes: - i) Manoxylic ii) Pycnoxylic The various classification of Gymnosperm proposed by various workers are as follows: - i) Sahni (1920): - He recognized two sub-divison in gymnosperm: - a) Phylospermae b) Stachyospermae BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants ii) Classification proposed by chamber lain (1934): - He divided Gymnosperm into two divisions: - a) Cycadophyta b) Coniterophyta iii) Classification proposed by Tippo (1942):- He considered Gymnosperm as a class of the sub- phylum pteropsida and divided them into two sub classes:- a) Cycadophyta b) Coniferophyta iv) D. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -
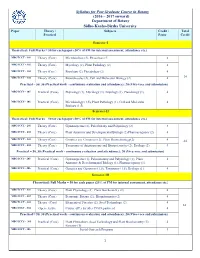
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Syllabus M.Sc. Botany (Choice Based Credit System)
Syllabus M.Sc. Botany (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD Page 1 of 21 DEPARTMENT OF BOTANY UNIVERSITY OF ALLAHABAD M.Sc. Syllabus (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) Semester – I Course Code Marks Course Title Credits BOT501 100 Phycology and Limnology 3 BOT502 100 Mycology and Plant Pathology 3 BOT503 100 Bryology and Pteridology 3 BOT504 100 Gymnosperm and Palaeobotany 3 BOT531 100 Lab Work I (based on Course BOT501 and BOT502) 4 (Excursion/field work/ Project) BOT532 100 Lab Work II (based on Course BOT503 and BOT504) 4 (Excursion/field work/Project) Total credits 20 Semester – II Course Code Marks Course Title Credits BOT505 100 Plant Morphology and Anatomy 3 BOT506 100 Reproductive Biology, Morphogenesis and Tissue culture 3 BOT507 100 Taxonomy of Angiosperm and Economic Botany 3 BOT508 100 Ecology and Phytogeography 3 BOT533 100 Lab Work III (based on Course BOT505 and BOT506) 4 (Field work/ Project) BOT534 100 Lab Work IV (based on Course BOT507 and BOT508) 4 (Field work/ Project) Total credits 20 Semester – III Course Code Marks Course Title Credits BOT601 100 Plant Physiology 3 BOT602 100 Plant Biochemistry and Biochemical Techniques 3 BOT603 100 Cytogenetics, Plant Breeding and Biostatistics 3 BOT604 100 Microbiology 3 BOT631 100 Lab Work V (based on Course BOT601 and BOT602) 4 BOT632 100 Lab Work VI (based on Course BOT603 and BOT604) 4 Total credits 20 Semester – IV Course Code Marks Course -

A Reappraisal of Mississippian (Tournaisian and Visean) Adpression Floras from Central and Northwestern Europe
Zitteliana A 54 (2014) 39 A reappraisal of Mississippian (Tournaisian and Visean) adpression floras from central and northwestern Europe Maren Hübers1, Benjamin Bomfleur2*, Michael Krings3, Christian Pott2 & Hans Kerp1 1Forschungsstelle für Paläobotanik am Geologisch-Paläontologischen Institut, Westfälische Zitteliana A 54, 39 – 52 Wilhelms-Universität Münster, Heisenbergstraße 2, 48149 Münster, Germany München, 31.12.2014 2Swedish Museum of Natural History, Department of Paleobiology, Box 50007, SE-104 05, Stockholm, Sweden Manuscript received 3Department für Geo- und Umweltwissenschaften, Paläontologie und Geobiologie, Ludwig- 01.02.2014; revision Maximilians-Universität, and Bayerische Staatssammlung für Paläontologie und Geologie, Richard- accepted 07.04.2014 Wagner-Straße 10, 80333 Munich, Germany ISSN 1612 - 412X *Author for correspondence and reprint requests: E-mail: [email protected] Abstract Mississippian plant fossils are generally rare, and in central and northwestern Europe especially Tournaisian to middle Visean fossil floras are restricted to isolated occurrences. While sphenophytes and lycophytes generally are represented by only a few widespread and long-ranging taxa such as Archaeocalamites radiatus, Sphenophyllum tenerrimum and several species of Lepidodendropsis and Lepido- dendron, Visean floras in particular show a remarkably high diversity of fern-like foliage, including filiform types (Rhodea, Diplotmema), forms with bipartite fronds (Sphenopteridium, Diplopteridium, Spathulopteris, Archaeopteridium), others with monopodial, pinnate fronds (Anisopteris, Fryopsis) and still others characterized by several-times pinnate fronds (e.g., Adiantites, Triphyllopteris, Sphenopteris, Neu- ropteris). Most of these leaf types have been interpreted as belonging to early seed ferns, whereas true ferns seem to have been rare or lacking in impression/compression floras. In the upper Visean, two types of plant assemblages can be distinguished, i.e., the northern Kohlenkalk-type and the south-eastern Kulm-type assemblage. -
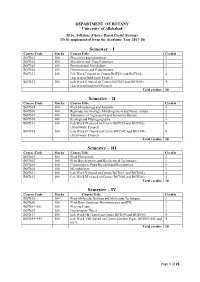
Semester – I Semester – II Semester – III Semester – IV
DEPARTMENT OF BOTANY University of Allahabad M.Sc. Syllabus (Choice Based Credit System) (To be implemented from the Academic Year 2017-18) Semester – I Course Code Marks Course Title Credits BOT501 100 Phycology and Limnology 3 BOT502 100 Mycology and Plant Pathology 3 BOT503 100 Bryology and Pteridology 3 BOT504 100 Gymnosperm and Palaeobotany 3 BOT531 100 Lab Work I (based on Course BOT501 and BOT502) 4 (Excursion/field work/ Project) BOT532 100 Lab Work II (based on Course BOT503 and BOT504) 4 (Excursion/field work/Project) Total credits 20 Semester – II Course Code Marks Course Title Credits BOT505 100 Plant Morphology and Anatomy 3 BOT506 100 Reproductive Biology, Morphogenesis and Tissue culture 3 BOT507 100 Taxonomy of Angiosperm and Economic Botany 3 BOT508 100 Ecology and Phytogeography 3 BOT533 100 Lab Work III (based on Course BOT505 and BOT506) 4 (Field work/ Project) BOT534 100 Lab Work IV (based on Course BOT507 and BOT508) 4 (Field work/ Project) Total credits 20 Semester – III Course Code Marks Course Title Credits BOT601 100 Plant Physiology 3 BOT602 100 Plant Biochemistry and Biochemical Techniques 3 BOT603 100 Cytogenetics, Plant Breeding and Biostatistics 3 BOT604 100 Microbiology 3 BOT631 100 Lab Work V (based on Course BOT601 and BOT602) 4 BOT632 100 Lab Work VI (based on Course BOT603 and BOT604) 4 Total credits 20 Semester – IV Course Code Marks Course Title Credits BOT605 100 Plant Molecular Biology and Molecular Techniques 3 BOT606 100 Plant Biotechnology, Bioinformatics and IPR 3 BOT651-662 100 Elective Paper -

An Overview of the Fossil Record of Climbers: Bejucos, Sogas, Trepadoras, Lianas, Cipós, and Vines
Rev. bras. paleontol. 12(2):149-160, Maio/Agosto 2009 © 2009 by the Sociedade Brasileira de Paleontologia doi:10.4072/rbp.2009.2.05 AN OVERVIEW OF THE FOSSIL RECORD OF CLIMBERS: BEJUCOS, SOGAS, TREPADORAS, LIANAS, CIPÓS, AND VINES ROBYN J. BURNHAM Museum of Paleontology, University of Michigan 1109 Geddes Avenue, Ann Arbor, MI 48109-1079, USA. [email protected] ABSTRACT – One of the most obvious life forms in tropical forests today is the liana, which laces together tree canopies and climbs the dark interiors of forests with snake-like stems. Lianas are ecologically important in extant, forested ecosystems, both intact and disturbed. Their contribution to forest diversity, food resources, structural complexity, and plant-animal interactions are recognized, but rarely studied. Climbers (woody lianas and herbaceous vines) are viewed as everything from diversity contributors to forest growth inhibitors by modern ecologists and systematists. Climbers take advantage of the structural support of trees to invest proportionately more in vegetative and reproductive organs, resulting in proliferation at the individual and species level. Today the climbing habit is dominated by angiosperm species, with only a minor contribution from ferns plus a single non-angiosperm seed plant genus, Gnetum. This contribution reports the establishment of the newly established database, Fossil Record of Climbers (FRC) that documents more than 1100 records of climbing plants from the Paleozoic to the Quaternary using published literature on the fossil record. The diversity of climbers in the fossil record prior to the evolution of angiosperms is explored, posing the hypothesis that climbers of the past had a similarly important role in tropical forests, at least in the Paleozoic. -

A Factor Analysis Approach to Modelling the Early Diversification of Terrestrial Vegetation E
A factor analysis approach to modelling the early diversification of terrestrial vegetation E. Capel, C. J. Cleal, P. Gerrienne, T. Servais, B. Cascales-Miñana To cite this version: E. Capel, C. J. Cleal, P. Gerrienne, T. Servais, B. Cascales-Miñana. A factor analysis approach to modelling the early diversification of terrestrial vegetation. Palaeogeography, Palaeoclimatology, Palaeoecology, Elsevier, 2021, 566, pp.110170. 10.1016/j.palaeo.2020.110170. hal-03331136 HAL Id: hal-03331136 https://hal.archives-ouvertes.fr/hal-03331136 Submitted on 1 Sep 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A factor analysis approach to modelling the early diversification of terrestrial vegetation E. Capel1, C.J. Cleal2, P. Gerrienne3†, T. Servais4, B. Cascales-Miñana4* 1 Univ. Lille, CNRS, UMR 8198 - Evo-Eco-Paleo, F-59000 Lille, France (eliott.capel@univ- lille.fr) 2 Department of Natural Sciences, National Museum Wales, Cardiff CF10 3NP, UK ([email protected]) 3 EDDy lab/Palaeobotany and Palaeopalynology, Univ. Liege, 4000 Liege, Belgium 4 CNRS, Univ. Lille, UMR 8198 - Evo-Eco-Paleo, F-59000 Lille, France ([email protected], [email protected]) *Corresponding author †Deceased 1 Abstract Data from a new comprehensive macrofossil-based compilation of early plant genera are analyzed via a Q-mode factor analysis. -

Gymnosperms from the Middle Triassic of Antarctica: the First Structurally Preserved Cycad Pollen Cone
Int. J. Plant Sci. 164(6):1007–1020. 2003. ᭧ 2003 by The University of Chicago. All rights reserved. 1058-5893/2003/16406-0016$15.00 GYMNOSPERMS FROM THE MIDDLE TRIASSIC OF ANTARCTICA: THE FIRST STRUCTURALLY PRESERVED CYCAD POLLEN CONE Sharon D. Klavins,* Edith L. Taylor,* Michael Krings,† and Thomas N. Taylor* *Department of Ecology and Evolutionary Biology and Natural History Museum and Biodiversity Research Center, University of Kansas, Lawrence, Kansas 66045-7534, U.S.A.; and †Bayerische Staatssammlung fu¨r Pala¨ontologie und Geologie, Funktionseinheit Pala¨ontologie, Richard-Wagner-Strasse 10, 80333 Munich, Germany The first permineralized cycad pollen cone is described from the lower Middle Triassic of Antarctica. The cone is characterized by helically arranged, wedge-shaped microsporophylls, each with five or more spinelike projections extending from the rhomboid distal face. The vascular cylinder is dissected and produces paired traces to each microsporophyll. Three vascular bundles enter the base of the microsporophyll and divide to produce at least five vascular strands in the sporophyll lamina. Pollen sacs occur in two radial clusters near the lateral margins on the abaxial surface of the microsporophyll. Each cluster bears up to eight elongate pollen sacs that are fused for approximately half their length and display longitudinal dehiscence. Pollen sacs are sessile and attached to a vascularized, receptacle-like pad of tissue that is raised from the surface of the microsporophyll. Pollen is ovoid, psilate, and monosulcate. Although the affinities of this cone with the Cycadales are obvious, the complement of characters in the fossil is unique and thus does not permit assignment to an extant family. -

MINUTES of the BOARD of ALDERMEN REGULAR MEETING of MARCH 21, 2017 Page I Of5
MINUTES OF THE BOARD OF ALDERMEN REGULAR MEETING OF MARCH 21, 2017 Page I of5 1. CALL TO ORDER A regular meeting of the Board of Aldermen was convened at 7:00 p.m. on Tuesday, March 21, 2017, at City Hall located at 8880 Clark Avenue, Parkville, Missouri, and was called to order by Mayor Nanette K. Johnston. City Clerk Melissa McChesney called the roll as follows: Ward I Alderman Diane Driver - present Ward 1 Alderman Tina Welch - present Ward 2 Alderman Jim Werner - present Ward 2 Alderman Dave Rittman - present Ward 3 Alderman Robert Lock - present Ward 3 Alderman Douglas Wylie - absent with prior notice Ward 4 Alderman Marc Sportsman - present Ward 4 Alderman Greg Plumb - present A quorum of the Board of Aldermen was present. The following staff was also present: City Administrator Joe Parente Kevin Chrisman, Police Chief Alysen Abel, Public Works Director Stephen Lachky, Community Development Director Matthew Chapman, Finance/Human Resources Director Tim Blakeslee, Assistant to the City Administrator Chris Williams, City Attorney Mayor Johnston led the Board in the Pledge of Allegiance to the Flag of the United States of America. Mayor Johnston observed a moment of silence for Toni Anderson. 2. CITIZEN INPUT A. Park University Fossil Presentation Scott Hageman, Tim Northcutt, Patty Ryberg and Brian Hoffman from Park University presented information about a new fossil that was found by amateur paleontologist Tim Northcutt on university property. A handout was distributed during the meeting attached as Exhibit A. They stated that Parkvillia northcutti was considered to be a link between conifers and fems and the earliest evidence of seeds. -

Reproductive Biology of Plants
K21132 6000 Broken Sound Parkway, NW Suite 300, Boca Raton, FL 33487 711 Third Avenue New York, NY 10017 an informa business 2 Park Square, Milton Park A SCIENCE PUBLISHERS BOOK www.crcpress.com Abingdon, Oxon OX14 4RN, UK About the pagination of this eBook Due to the unique page numbering scheme of this book, the electronic pagination of the eBook does not match the pagination of the printed version. To navigate the text, please use the electronic Table of Contents that appears alongside the eBook or the Search function. For citation purposes, use the page numbers that appear in the text. Reproductive Biology of Plants Reproductive Biology of Plants Editors K.G. Ramawat Former Professor & Head Botany Department, M.L. Sukhadia University Udaipur, India Jean-Michel Mérillon Université de Bordeaux Institut des Sciences de la Vigne et du Vin Villenave d’Ornon, France K.R. Shivanna Former Professor & Head Botany Department, University of Delhi Delhi, India p, A SCIENCE PUBLISHERS BOOK GL--Prelims with new title page.indd ii 4/25/2012 9:52:40 AM CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2014 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Version Date: 20140117 International Standard Book Number-13: 978-1-4822-0133-8 (eBook - PDF) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use.