Vulvovaginal Candidiasis (VVC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Viamet Reports Positive Results from Interim Analysis of REVIVE Phase 2B Trial of VT-1161 in Recurrent Vulvovaginal Candidiasis
Viamet Reports Positive Results from Interim Analysis of REVIVE Phase 2b Trial of VT-1161 in Recurrent Vulvovaginal Candidiasis -Interim Results Demonstrate Strong Clinical Benefit and Favorable Safety Profile- -Final Data Expected in Fourth Quarter of 2016- RESEARCH TRIANGLE PARK, N.C., March 9, 2016, – Viamet Pharmaceuticals, Inc. today reported positive results from a planned interim analysis of REVIVE (Recurrent Vulvovaginal Candidiasis Inhibition: an Oral VT-1161 Tablet Evaluation), its ongoing Phase 2b clinical trial of VT-1161 for the treatment of recurrent vulvovaginal candidiasis, or RVVC. RVVC is defined as three or more episodes of acute vulvovaginal candidiasis, or AVVC (commonly referred to as a vaginal yeast infection), in a 12-month period. VT-1161, the company’s lead product candidate, is a highly potent and selective, orally available inhibitor of fungal CYP51. “It is estimated that RVVC affects 5% to 8% of U.S. women during their child-bearing years and has a significant negative impact on quality of life,” commented Robert Schotzinger, M.D., Ph.D., CEO of Viamet. “Despite the number of women affected by this disease, we are not aware of any approved therapies for RVVC in the U.S. VT-1161 has demonstrated a high degree of potency against Candida species, the causative fungal pathogens responsible for RVVC, a robust oral pharmacokinetic profile, and a favorable safety profile in previous studies. These attributes, coupled with the positive interim results from our REVIVE trial, suggest that VT-1161 has the potential to be a highly effective treatment option for patients with RVVC.” REVIVE is a randomized, double-blind, placebo-controlled, 48-week clinical trial of VT-1161 in patients with RVVC. -

Candida Auris
microorganisms Review Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities Suhail Ahmad * and Wadha Alfouzan Department of Microbiology, Faculty of Medicine, Kuwait University, P.O. Box 24923, Safat 13110, Kuwait; [email protected] * Correspondence: [email protected]; Tel.: +965-2463-6503 Abstract: Candida auris, a recently recognized, often multidrug-resistant yeast, has become a sig- nificant fungal pathogen due to its ability to cause invasive infections and outbreaks in healthcare facilities which have been difficult to control and treat. The extraordinary abilities of C. auris to easily contaminate the environment around colonized patients and persist for long periods have recently re- sulted in major outbreaks in many countries. C. auris resists elimination by robust cleaning and other decontamination procedures, likely due to the formation of ‘dry’ biofilms. Susceptible hospitalized patients, particularly those with multiple comorbidities in intensive care settings, acquire C. auris rather easily from close contact with C. auris-infected patients, their environment, or the equipment used on colonized patients, often with fatal consequences. This review highlights the lessons learned from recent studies on the epidemiology, diagnosis, pathogenesis, susceptibility, and molecular basis of resistance to antifungal drugs and infection control measures to combat the spread of C. auris Citation: Ahmad, S.; Alfouzan, W. Candida auris: Epidemiology, infections in healthcare facilities. Particular emphasis is given to interventions aiming to prevent new Diagnosis, Pathogenesis, Antifungal infections in healthcare facilities, including the screening of susceptible patients for colonization; the Susceptibility, and Infection Control cleaning and decontamination of the environment, equipment, and colonized patients; and successful Measures to Combat the Spread of approaches to identify and treat infected patients, particularly during outbreaks. -

Candida Species Identification by NAA
Candida Species Identification by NAA Background Vulvovaginal candidiasis (VVC) occurs as a result of displacement of the normal vaginal flora by species of the fungal genus Candida, predominantly Candida albicans. The usual presentation is irritation, itching, burning with urination, and thick, whitish discharge.1 VVC accounts for about 17% to 39% of vaginitis1, and most women will be diagnosed with VVC at least once during their childbearing years.2 In simplistic terms, VVC can be classified into uncomplicated or complicated presentations. Uncomplicated VVC is characterized by infrequent symptomatic episodes, mild to moderate symptoms, or C albicans infection occurring in nonpregnant and immunocompetent women.1 Complicated VVC, in contrast, is typified by severe symptoms, frequent recurrence, infection with Candida species other than C albicans, and/or occurrence during pregnancy or in women with immunosuppression or other medical conditions.1 Diagnosis and Treatment of VVC Traditional diagnosis of VVC is accomplished by either: (i) direct microscopic visualization of yeast-like cells with or without pseudohyphae; or (ii) isolation of Candida species by culture from a vaginal sample.1 Direct microscopy sensitivity is about 50%1 and does not provide a species identification, while cultures can have long turnaround times. Today, nucleic acid amplification-based (NAA) tests (eg, PCR) for Candida species can provide high-quality diagnostic information with quicker turnaround times and can also enable investigation of common potential etiologies -

Candida & Nutrition
Candida & Nutrition Presented by: Pennina Yasharpour, RDN, LDN Registered Dietitian Dickinson College Kline Annex Email: [email protected] What is Candida? • Candida is a type of yeast • Most common cause of fungal infections worldwide Candida albicans • Most common species of candida • C. albicans is part of the normal flora of the mucous membranes of the respiratory, gastrointestinal and female genital tracts. • Causes infections Candidiasis • Overgrowth of candida can cause superficial infections • Commonly known as a “yeast infection” • Mouth, skin, stomach, urinary tract, and vagina • Oropharyngeal candidiasis (thrush) • Oral infections, called oral thrush, are more common in infants, older adults, and people with weakened immune systems • Vulvovaginal candidiasis (vaginal yeast infection) • About 75% of women will get a vaginal yeast infection during their lifetime Causes of Candidiasis • Humans naturally have small amounts of Candida that live in the mouth, stomach, and vagina and don't cause any infections. • Candidiasis occurs when there's an overgrowth of the fungus RISK FACTORS WEAKENED ASSOCIATED IMUMUNE SYSTEM FACTORS • HIV/AIDS (Immunosuppression) • Infants • Diabetes • Elderly • Corticosteroid use • Antibiotic use • Contraceptives • Increased estrogen levels Type 2 Diabetes – Glucose in vaginal secretions promote Yeast growth. (overgrowth) Treatment • Antifungal medications • Oral rinses and tablets, vaginal tablets and suppositories, and creams. • For vaginal yeast infections, medications that are available over the counter include creams and suppositories, such as miconazole (Monistat), ticonazole (Vagistat), and clotrimazole (Gyne-Lotrimin). • Your doctor may prescribe a pill, fluconazole (Diflucan). The Candida Diet • Avoid carbohydrates: Supporters believe that Candida thrives on simple sugars and recommend removing them, along with low-fiber carbohydrates (eg, white bread). -

Fungi in Bronchiectasis: a Concise Review
International Journal of Molecular Sciences Review Fungi in Bronchiectasis: A Concise Review Luis Máiz 1, Rosa Nieto 1 ID , Rafael Cantón 2 ID , Elia Gómez G. de la Pedrosa 2 and Miguel Ángel Martinez-García 3,* ID 1 Servicio de Neumología, Unidad de Bronquiectasias y Fibrosis Quística, Hospital Universitario Ramón y Cajal, 28034 Madrid, Spain; [email protected] (L.M.); [email protected] (R.N.) 2 Servicio de Microbiología, Hospital Universitario Ramón y Cajal and Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), 28034 Madrid, Spain; [email protected] (R.C.); [email protected] (E.G.G.d.l.P.) 3 Servicio de Neumología, Hospital Universitario y Politécnico la Fe, 46016 Valencia, Spain * Correspondence: [email protected]; Tel.: +34-60-986-5934 Received: 3 December 2017; Accepted: 31 December 2017; Published: 4 January 2018 Abstract: Although the spectrum of fungal pathology has been studied extensively in immunosuppressed patients, little is known about the epidemiology, risk factors, and management of fungal infections in chronic pulmonary diseases like bronchiectasis. In bronchiectasis patients, deteriorated mucociliary clearance—generally due to prior colonization by bacterial pathogens—and thick mucosity propitiate, the persistence of fungal spores in the respiratory tract. The most prevalent fungi in these patients are Candida albicans and Aspergillus fumigatus; these are almost always isolated with bacterial pathogens like Haemophillus influenzae and Pseudomonas aeruginosa, making very difficult to define their clinical significance. Analysis of the mycobiome enables us to detect a greater diversity of microorganisms than with conventional cultures. The results have shown a reduced fungal diversity in most chronic respiratory diseases, and that this finding correlates with poorer lung function. -

Managing Athlete's Foot
South African Family Practice 2018; 60(5):37-41 S Afr Fam Pract Open Access article distributed under the terms of the ISSN 2078-6190 EISSN 2078-6204 Creative Commons License [CC BY-NC-ND 4.0] © 2018 The Author(s) http://creativecommons.org/licenses/by-nc-nd/4.0 REVIEW Managing athlete’s foot Nkatoko Freddy Makola,1 Nicholus Malesela Magongwa,1 Boikgantsho Matsaung,1 Gustav Schellack,2 Natalie Schellack3 1 Academic interns, School of Pharmacy, Sefako Makgatho Health Sciences University 2 Clinical research professional, pharmaceutical industry 3 Professor, School of Pharmacy, Sefako Makgatho Health Sciences University *Corresponding author, email: [email protected] Abstract This article is aimed at providing a succinct overview of the condition tinea pedis, commonly referred to as athlete’s foot. Tinea pedis is a very common fungal infection that affects a significantly large number of people globally. The presentation of tinea pedis can vary based on the different clinical forms of the condition. The symptoms of tinea pedis may range from asymptomatic, to mild- to-severe forms of pain, itchiness, difficulty walking and other debilitating symptoms. There is a range of precautionary measures available to prevent infection, and both oral and topical drugs can be used for treating tinea pedis. This article briefly highlights what athlete’s foot is, the different causes and how they present, the prevalence of the condition, the variety of diagnostic methods available, and the pharmacological and non-pharmacological management of the -
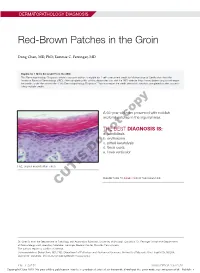
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

Oral Thrush A
Oral thrush A. Introduction Oral thrush occurs when a yeast infection develops on the inside of your mouth and on your tongue. This condition is also known as oral candidiasis, oropharyngeal candidiasis, or, simply, thrush. The Candida albicans (C. albicans) fungus causes oral thrush. Candida is a normal organism in your mouth, but sometimes it can overgrow and cause symptoms. Oral thrush most often occurs in infants and toddlers. Oral thrush is typically mild and rarely causes complications. However, the condition can be problematic for those with weakened immune systems. B. Signs and Symtoms Oral thrush is usually harmless. It's common in babies and older people with dentures. It can be easily treated with medicines bought from a pharmacy. Adults Your mouth is red inside and you have white patches When you wipe off the white patches, they leave red spots that can bleed Other symptoms in adults are: 1.cracks at the corners of the mouth 2.not tasting things properly 3.an unpleasant taste in the mouth 4.pain inside the mouth (for example, a sore tongue or sore gums) 5.difficulty eating and drinking Oral thrush in adults isn't contagious. Babies A white coating on the tongue like cottage cheese – this can't be rubbed off easily Sometimes there are white spots in their mout Other symptoms in babies are: 1.they don't want to feed 2.nappy rash Babies can pass oral thrush on through breastfeeding. This can cause nipple thrush in mothers. C. Treatment Treatment for oral thrush varies depending on your age and overall health. -

Fungal Infection
Fungal Infection What is a Fungus? A Fungus is a tiny microorganism that belongs to the eukaryotic group and is ubiquitous in nature. They can be found in the soil, plants, decaying organic matter, damp places, and also in humans and animals. They include yeast, moulds, mushrooms and several others out of which some are pathogenic to humans. What is Fungal Infection? Fungal infection, also known as mycosis, is a broad term to describe various disease conditions caused by different types of fungi. Sometimes, fungi that aren’t present in the normal flora can colonize and induce an infection. Or the disruption of normal flora can lead to a fungal infection. Fungal infections can affect anyone at any age. If it spreads, then the treatment and control of these infections become extremely challenging. What are the different types of Fungal Infections? There are several types of fungal infections that can cause serious damage to health. Some of the common types of fungal infections are as follows: Ringworm infections Nail infections Athlete’s foot Vaginal yeast infections Oral thrush Jock itch 1 Scalp infections, etc. The fungus can also cause a full-blown infection, sinus infections and deep lung infections. What are the signs and symptoms of different Fungal Infections? The signs and symptoms of different fungal infections widely vary from each other, and some of them are discussed below: Signs and symptoms of Ringworm infections- Formation of a red ring-like patch, development of blisters around the affected area, itching and burning sensation. Signs and symptoms of Nail infections- Discoloration of nails, destruction of their normal shape, brittle nails, a feeling of pain and pressure in the affected area. -

TISSUE INJURY INDUCED by CANDIDA ALBICANS Menstrual Period Occurring on the 26Th Day of "She's More Active," "Doesn't Sit and Hold Her Treatment
Tissue Injury Induced By Candida Albicans Mental and Neurologic Manifestations C. Orian Truss, M.D.I Introduction body complexes or the presence of "auto- Medical research of the past several decades antibodies" to one's own tissues are but two has greatly expanded our knowledge of the examples. deranged chemistry and physiology that The same "dead end" is reached, however, characterize many individual disease states. Yet when the search for a virus or other antigenic most diseases have stubbornly resisted the agent is unfruitful. The evidence indicates that massive effort to unlock the secret of their an immunologic response is taking place and etiologies. We learn more and more about the is in some way involved in the disease abnormalities of function that result in clinical process, but the antigenic agent continues to symptoms and signs, but continue to be frustrated elude discovery. in our effort to discover the identity of the factor Or we may postulate that the immunologic initiating the departure from normal function. It is process occurs secondary to toxic damage to a unfortunate that the list of such diseases is headed tissue, leading to autoantibodies and perhaps by those that are the most frequent cause of to antigen-antibody complex deposition. But disability and death. Among them would be again, such a toxin escapes detection, if it atherosclerosis, most often clinically expressed as exists. hypertension, heart attacks, and strokes; In presenting selected, cases treated during malignancies, "auto-immune" diseases, arthritis, the past 16 years, I hope to call attention to muscle-wasting diseases, mental and neurologic Candida Albicans as a possible candidate for disorders, and many others. -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

(Clotrimazole Vaginal Cream USP 1%) Clotrimaderm Vaginal 3
PRODUCT MONOGRAPH Clotrimaderm Vaginal 6 (Clotrimazole Vaginal Cream USP 1%) Clotrimaderm Vaginal 3 (Clotrimazole Vaginal Cream USP 2%) Clotrimaderm External Cream (Clotrimazole Vaginal Cream USP 1%) Antifungal Agent Taro Pharmaceuticals Inc. Date of Preparation: 130 East Drive January 27, 2020 Brampton, Ontario L6T 1C1 Control #232921 Page 1 of 22 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION ................................................................ 3 SUMMARY PRODUCT INFORMATION ...................................................................................... 3 INDICATIONS AND CLINICAL USE ........................................................................................... 3 CONTRAINDICATIONS ................................................................................................................. 3 WARNINGS AND PRECAUTIONS ............................................................................................... 3 ADVERSE REACTIONS ................................................................................................................. 4 DRUG INTERACTIONS .................................................................................................................. 5 DOSAGE AND ADMINISTRATION ............................................................................................. 5 OVERDOSAGE ................................................................................................................................. 6 ACTION AND CLINICAL PHARMACOLOGY ......................................................................