Primepcr™Assay Validation Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulation and Dysregulation of Chromosome Structure in Cancer
Regulation and Dysregulation of Chromosome Structure in Cancer The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Hnisz, Denes et al. “Regulation and Dysregulation of Chromosome Structure in Cancer.” Annual Review of Cancer Biology 2, 1 (March 2018): 21–40 © 2018 Annual Reviews As Published https://doi.org/10.1146/annurev-cancerbio-030617-050134 Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/117286 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ Regulation and dysregulation of chromosome structure in cancer Denes Hnisz1*, Jurian Schuijers1, Charles H. Li1,2, Richard A. Young1,2* 1 Whitehead Institute for Biomedical Research, 455 Main Street, Cambridge, MA 02142, USA 2 Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA * Corresponding authors Corresponding Authors: Denes Hnisz Whitehead Institute for Biomedical Research 455 Main Street Cambridge, MA 02142 Tel: (617) 258-7181 Fax: (617) 258-0376 [email protected] Richard A. Young Whitehead Institute for Biomedical Research 455 Main Street Cambridge, MA 02142 Tel: (617) 258-5218 Fax: (617) 258-0376 [email protected] 1 Summary Cancer arises from genetic alterations that produce dysregulated gene expression programs. Normal gene regulation occurs in the context of chromosome loop structures called insulated neighborhoods, and recent studies have shown that these structures are altered and can contribute to oncogene dysregulation in various cancer cells. We review here the types of genetic and epigenetic alterations that influence neighborhood structures and contribute to gene dysregulation in cancer, present models for insulated neighborhoods associated with the most prominent human oncogenes, and discuss how such models may lead to further advances in cancer diagnosis and therapy. -

DNA Replication Stress Response Involving PLK1, CDC6, POLQ
DNA replication stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients C. Allera-Moreau, I. Rouquette, B. Lepage, N. Oumouhou, M. Walschaerts, E. Leconte, V. Schilling, K. Gordien, L. Brouchet, Mb Delisle, et al. To cite this version: C. Allera-Moreau, I. Rouquette, B. Lepage, N. Oumouhou, M. Walschaerts, et al.. DNA replica- tion stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients. Oncogenesis, Nature Publishing Group: Open Access Journals - Option C, 2012, 1, pp.e30. 10.1038/oncsis.2012.29. hal-00817701 HAL Id: hal-00817701 https://hal.archives-ouvertes.fr/hal-00817701 Submitted on 9 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Citation: Oncogenesis (2012) 1, e30; doi:10.1038/oncsis.2012.29 & 2012 Macmillan Publishers Limited All rights reserved 2157-9024/12 www.nature.com/oncsis ORIGINAL ARTICLE DNA replication stress response involving PLK1, CDC6, POLQ, RAD51 and CLASPIN upregulation prognoses the outcome of early/mid-stage non-small cell lung cancer patients C Allera-Moreau1,2,7, I Rouquette2,7, B Lepage3, N Oumouhou3, M Walschaerts4, E Leconte5, V Schilling1, K Gordien2, L Brouchet2, MB Delisle1,2, J Mazieres1,2, JS Hoffmann1, P Pasero6 and C Cazaux1 Lung cancer is the leading cause of cancer deaths worldwide. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

Investigation of the Underlying Hub Genes and Molexular Pathogensis in Gastric Cancer by Integrated Bioinformatic Analyses
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.20.423656; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Investigation of the underlying hub genes and molexular pathogensis in gastric cancer by integrated bioinformatic analyses Basavaraj Vastrad1, Chanabasayya Vastrad*2 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.20.423656; this version posted December 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract The high mortality rate of gastric cancer (GC) is in part due to the absence of initial disclosure of its biomarkers. The recognition of important genes associated in GC is therefore recommended to advance clinical prognosis, diagnosis and and treatment outcomes. The current investigation used the microarray dataset GSE113255 RNA seq data from the Gene Expression Omnibus database to diagnose differentially expressed genes (DEGs). Pathway and gene ontology enrichment analyses were performed, and a proteinprotein interaction network, modules, target genes - miRNA regulatory network and target genes - TF regulatory network were constructed and analyzed. Finally, validation of hub genes was performed. The 1008 DEGs identified consisted of 505 up regulated genes and 503 down regulated genes. -

Supplementary Table S1. Correlation Between the Mutant P53-Interacting Partners and PTTG3P, PTTG1 and PTTG2, Based on Data from Starbase V3.0 Database
Supplementary Table S1. Correlation between the mutant p53-interacting partners and PTTG3P, PTTG1 and PTTG2, based on data from StarBase v3.0 database. PTTG3P PTTG1 PTTG2 Gene ID Coefficient-R p-value Coefficient-R p-value Coefficient-R p-value NF-YA ENSG00000001167 −0.077 8.59e-2 −0.210 2.09e-6 −0.122 6.23e-3 NF-YB ENSG00000120837 0.176 7.12e-5 0.227 2.82e-7 0.094 3.59e-2 NF-YC ENSG00000066136 0.124 5.45e-3 0.124 5.40e-3 0.051 2.51e-1 Sp1 ENSG00000185591 −0.014 7.50e-1 −0.201 5.82e-6 −0.072 1.07e-1 Ets-1 ENSG00000134954 −0.096 3.14e-2 −0.257 4.83e-9 0.034 4.46e-1 VDR ENSG00000111424 −0.091 4.10e-2 −0.216 1.03e-6 0.014 7.48e-1 SREBP-2 ENSG00000198911 −0.064 1.53e-1 −0.147 9.27e-4 −0.073 1.01e-1 TopBP1 ENSG00000163781 0.067 1.36e-1 0.051 2.57e-1 −0.020 6.57e-1 Pin1 ENSG00000127445 0.250 1.40e-8 0.571 9.56e-45 0.187 2.52e-5 MRE11 ENSG00000020922 0.063 1.56e-1 −0.007 8.81e-1 −0.024 5.93e-1 PML ENSG00000140464 0.072 1.05e-1 0.217 9.36e-7 0.166 1.85e-4 p63 ENSG00000073282 −0.120 7.04e-3 −0.283 1.08e-10 −0.198 7.71e-6 p73 ENSG00000078900 0.104 2.03e-2 0.258 4.67e-9 0.097 3.02e-2 Supplementary Table S2. -

Statistical and Bioinformatic Analysis of Hemimethylation Patterns in Non-Small Cell Lung Cancer
Statistical and Bioinformatic Analysis of Hemimethylation Patterns in Non-Small Cell Lung Cancer Shuying Sun ( [email protected] ) Texas State University San Marcos https://orcid.org/0000-0003-3974-6996 Austin Zane Texas A&M University College Station Carolyn Fulton Schreiner University Jasmine Philipoom Case Western Reserve University Research article Keywords: Methylation, Hemimethylation, Lung Cancer, Bioinformatics, Epigenetics Posted Date: October 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-17794/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on March 12th, 2021. See the published version at https://doi.org/10.1186/s12885-021-07990-7. Page 1/29 Abstract Background: DNA methylation is an epigenetic event involving the addition of a methyl-group to a cytosine-guanine base pair (i.e., CpG site). It is associated with different cancers. Our research focuses on studying non- small cell lung cancer hemimethylation, which refers to methylation occurring on only one of the two DNA strands. Many studies often assume that methylation occurs on both DNA strands at a CpG site. However, recent publications show the existence of hemimethylation and its signicant impact. Therefore, it is important to identify cancer hemimethylation patterns. Methods: In this paper, we use the Wilcoxon signed rank test to identify hemimethylated CpG sites based on publicly available non-small cell lung cancer methylation sequencing data. We then identify two types of hemimethylated CpG clusters, regular and polarity clusters, and genes with large numbers of hemimethylated sites. -

Disruption of Chtf18 Causes Defective Meiotic Recombination in Male Mice
Disruption of Chtf18 Causes Defective Meiotic Recombination in Male Mice Karen M. Berkowitz1,2*, Aislinn R. Sowash1,2, Lydia R. Koenig1,2, Dawnette Urcuyo1,2, Fahmida Khan1,2, Fang Yang3, P. Jeremy Wang3, Thomas A. Jongens4, Klaus H. Kaestner4 1 Department of Obstetrics and Gynecology, Drexel University College of Medicine, Philadelphia, Pennsylvania, United States of America, 2 Department of Biochemistry and Molecular Biology, Drexel University College of Medicine, Philadelphia, Pennsylvania, United States of America, 3 Department of Animal Biology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 4 Department of Genetics, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America Abstract CHTF18 (chromosome transmission fidelity factor 18) is an evolutionarily conserved subunit of the Replication Factor C-like complex, CTF18-RLC. CHTF18 is necessary for the faithful passage of chromosomes from one daughter cell to the next during mitosis in yeast, and it is crucial for germline development in the fruitfly. Previously, we showed that mouse Chtf18 is expressed throughout the germline, suggesting a role for CHTF18 in mammalian gametogenesis. To determine the role of CHTF18 in mammalian germ cell development, we derived mice carrying null and conditional mutations in the Chtf18 gene. Chtf18-null males exhibit 5-fold decreased sperm concentrations compared to wild-type controls, resulting in subfertility. Loss of Chtf18 results in impaired spermatogenesis; spermatogenic cells display abnormal morphology, and the stereotypical arrangement of cells within seminiferous tubules is perturbed. Meiotic recombination is defective and homologous chromosomes separate prematurely during prophase I. Repair of DNA double-strand breaks is delayed and incomplete; both RAD51 and cH2AX persist in prophase I. -

Cryptic Splicing Events in the Iron Transporter ABCB7 and Other Key Target Genes in SF3B1-Mutant Myelodysplastic Syndromes
OPEN Leukemia (2016) 30, 2322–2331 www.nature.com/leu ORIGINAL ARTICLE Cryptic splicing events in the iron transporter ABCB7 and other key target genes in SF3B1-mutant myelodysplastic syndromes H Dolatshad1,2,8, A Pellagatti1,2,8, FG Liberante3, M Llorian4, E Repapi5, V Steeples1,2, S Roy1,2, L Scifo1,2, RN Armstrong1,2, J Shaw1,2, BH Yip1,2, S Killick6,RKušec7, S Taylor5, KI Mills3, KI Savage3, CWJ Smith4 and J Boultwood1,2 The splicing factor SF3B1 is the most frequently mutated gene in myelodysplastic syndromes (MDS), and is strongly associated with the presence of ring sideroblasts (RS). We have performed a systematic analysis of cryptic splicing abnormalities from RNA sequencing data on hematopoietic stem cells (HSCs) of SF3B1-mutant MDS cases with RS. Aberrant splicing events in many downstream target genes were identified and cryptic 3′ splice site usage was a frequent event in SF3B1-mutant MDS. The iron transporter ABCB7 is a well-recognized candidate gene showing marked downregulation in MDS with RS. Our analysis unveiled aberrant ABCB7 splicing, due to usage of an alternative 3′ splice site in MDS patient samples, giving rise to a premature termination codon in the ABCB7 mRNA. Treatment of cultured SF3B1-mutant MDS erythroblasts and a CRISPR/Cas9-generated SF3B1-mutant cell line with the nonsense-mediated decay (NMD) inhibitor cycloheximide showed that the aberrantly spliced ABCB7 transcript is targeted by NMD. We describe cryptic splicing events in the HSCs of SF3B1-mutant MDS, and our data support a model in which NMD-induced downregulation of the iron exporter ABCB7 mRNA transcript resulting from aberrant splicing caused by mutant SF3B1 underlies the increased mitochondrial iron accumulation found in MDS patients with RS. -

ATR16 Syndrome: Mechanisms Linking Monosomy to Phenotype 2 3 4 Christian Babbs,*1 Jill Brown,1 Sharon W
bioRxiv preprint doi: https://doi.org/10.1101/768895; this version posted October 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 ATR16 Syndrome: Mechanisms Linking Monosomy to Phenotype 2 3 4 Christian Babbs,*1 Jill Brown,1 Sharon W. Horsley,1 Joanne Slater,1 Evie Maifoshie,1 5 Shiwangini Kumar,2 Paul Ooijevaar,3 Marjolein Kriek,3 Amanda Dixon-McIver,2 6 Cornelis L. Harteveld,3 Joanne Traeger-Synodinos,4 Douglas Higgs1 and Veronica 7 Buckle*1 8 9 10 11 12 1. MRC Molecular Haematology Unit, MRC Weatherall Institute of Molecular 13 Medicine, University of Oxford, Oxford, UK. 14 15 2. IGENZ Ltd, Auckland, New Zealand. 16 17 3. Department of Clinical Genetics, Leiden University Medical Center, Leiden, The 18 Netherlands. 19 20 4. Department of Medical Genetics, National and Kapodistrian University of Athens, 21 Greece. 22 23 24 25 *Authors for correspondence 26 [email protected] 27 [email protected] 28 29 1 bioRxiv preprint doi: https://doi.org/10.1101/768895; this version posted October 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Abstract 2 3 Background: Sporadic deletions removing 100s-1000s kb of DNA, and 4 variable numbers of poorly characterised genes, are often found in patients 5 with a wide range of developmental abnormalities. In such cases, 6 understanding the contribution of the deletion to an individual’s clinical 7 phenotype is challenging. -

Systematic Genome Editing of the Genes on Zebrafish Chromosome 1 by CRISPR/Cas9
Downloaded from genome.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press Resource Systematic genome editing of the genes on zebrafish Chromosome 1 by CRISPR/Cas9 Yonghua Sun,1 Bo Zhang,2 Lingfei Luo,3 De-Li Shi,4 Han Wang,5 Zongbin Cui,1 Honghui Huang,3 Ying Cao,6 Xiaodong Shu,7 Wenqing Zhang,8 Jianfeng Zhou,9 Yun Li,9 Jiulin Du,10 Qingshun Zhao,11 Jun Chen,12 Hanbing Zhong,13 Tao P. Zhong,14 Li Li,3 Jing-Wei Xiong,15 Jinrong Peng,12 Wuhan Xiao,1 Jian Zhang,16 Jihua Yao,17 Zhan Yin,1 Xianming Mo,18 Gang Peng,19 Jun Zhu,20 Yan Chen,21 Yong Zhou,22 Dong Liu,13 Weijun Pan,22 Yiyue Zhang,8 Hua Ruan,3 Feng Liu,23 Zuoyan Zhu,1 Anming Meng,24 and The ZAKOC Consortium25 1State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Innovation Academy for Seed Design, Chinese Academy of Sciences, Wuhan, Hubei, 430072, China; 2Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education, Peking University Genome Editing Research Center, College of Life Sciences, Peking University, Beijing, 100871, China; 3School of Life Sciences, Southwest University, Chongqing, 400715, China; 4Guangdong Medical University, Zhanjiang, Guangdong, 524023, China; 5Center for Circadian Clocks, Soochow University, Suzhou, Jiangsu, 215123, China; 6School of Life Sciences and Technology, Tongji University, Shanghai, 200092, China; 7Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, Guangdong, 510530, China; 8Division of Cell, Developmental and Integrative -

DNA Replication and Sister Chromatid Cohesion 1 (DSCC1)
Journal of Cancer 2019, Vol. 10 6142 Ivyspring International Publisher Journal of Cancer 2019; 10(24): 6142-6153. doi: 10.7150/jca.32339 Research Paper DNA Replication and Sister Chromatid Cohesion 1 (DSCC1) of the Replication Factor Complex CTF18- RFC is Critical for Colon Cancer Cell Growth Jong-Tae Kim1*, Hee Jun Cho1*, Sang Yoon Park1, Byung Moo Oh1,2, Yo Sep Hwang1,2, Kyoung Eun Baek1, Young-Ha Lee3, Hee Cheol Kim4 and Hee Gu Lee1,2 1. Immunotherapy Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Republic of Korea. 2. Department of Biomolecular Science, University of Science and Technology (UST), Daejeon, Republic of Korea. 3. Department of Infection Biology, Chungnam National University School of Medicine, Daejeon, Republic of Korea. 4. Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. *These authors contributed equally to this work. Corresponding authors: Hee Cheol Kim, M.D., Ph.D., Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. E-mail: [email protected] or Hee Gu Lee, Ph.D., Immunotherapy Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 34141, Republic of Korea. Tel: +82-42-860-4182; Fax: +82-42-860-4593; E-mail: [email protected] © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2018.12.17; Accepted: 2019.08.26; Published: 2019.10.15 Abstract DNA replication and sister chromatid cohesion 1 (DSCC1) combines with chromosome transmission-fidelity protein 18 (CTF18) to form a CTF18-DSCC1-CTF8 (CTF18-1-8) module, which in combination with CTF18-replication factor C (RFC) acts as a proliferating cell nuclear antigen (PCNA) loader during DNA replication-associated processes. -
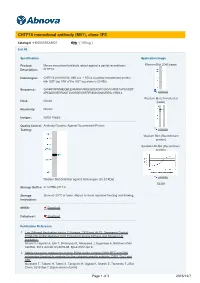
CHTF18 Monoclonal Antibody (M01), Clone 1F5
CHTF18 monoclonal antibody (M01), clone 1F5 Catalog # : H00063922-M01 規格 : [ 100 ug ] List All Specification Application Image Product Mouse monoclonal antibody raised against a partial recombinant Western Blot (Cell lysate) Description: CHTF18. Immunogen: CHTF18 (AAH18184, 886 a.a. ~ 975 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Sequence: GVHRPAPRNHEQRLEHIMRRAAREEQPEKDFFGRVVVRSTAVPSAGDT APEQDSVERRMGTAVGRSEVWFRFNEGVSNAVRRSLYIRDLL enlarge Western Blot (Transfected Host: Mouse lysate) Reactivity: Human Isotype: IgG2a Kappa Quality Control Antibody Reactive Against Recombinant Protein. Testing: enlarge Western Blot (Recombinant protein) Sandwich ELISA (Recombinant protein) enlarge Western Blot detection against Immunogen (35.53 KDa) . ELISA Storage Buffer: In 1x PBS, pH 7.4 Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Datasheet: Download Publication Reference 1. Two Different Replication Factor C Proteins, Ctf18 and RFC1, Separately Control PCNA-CRL4Cdt2-Mediated Cdt1 Proteolysis during S Phase and following UV Irradiation. Shiomi Y, Hayashi A, Ishii T, Shinmyozu K, Nakayama J, Sugasawa K, Nishitani H.Mol Cell Biol. 2012 Jun;32(12):2279-88. Epub 2012 Apr 9. 2. Stable interaction between the human PCNA loader complex Ctf18-RFC and DNA polymerase {epsilon} is mediated by the cohesion specific subunits, Ctf18, Dcc1 and Ctf8. Murakami T, Takano R, Takeo S, Taniguchi R, Ogawa K, Ohashi E, Tsurimoto T.J Biol Chem. 2010 Sep 7. [Epub ahead of print] Page 1 of 3 2016/12/7 Applications Western Blot (Cell lysate) CHTF18 monoclonal antibody (M01), clone 1F5 Western Blot analysis of CHTF18 expression in HeLa ( Cat # L013V1 ). Protocol Download Western Blot (Transfected lysate) Western Blot analysis of CHTF18 expression in transfected 293T cell line by CHTF18 monoclonal antibody (M01), clone 1F5.