Short Title for Use on Binding the Use of Objective Tests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

POUR APP Liste Tournée 2014 Rev3 Cindy
OPUS PHOTO ID TOUR RENEWAL IN SCHOOLS Schedule subject to change Please check this list again to confirm the photo session date in your school. School Tour Date A Academie Dunton 15-sept Académie Michèle-Provost 08-oct Académie Roberval 07-oct B Beaconsfield High School 09-sept Beurling Academy 25-sept Bialik High School 14-oct C CAE d’Outremont (prise de photo à l'école Saint-Laurent édifice Cardinal) 15-oct Cégep André-Laurendeau 19-sept Cégep de Saint-Laurent 17-sept Cégep du Vieux Montréal 11-12 sept Cégep Gérald-Godin 08-sept Cégep Marie-Victorin 10-sept Centennial Academy 08-oct Centennial College (prise de photo à Centennial Academy) 08-oct Centre Champagnat 07-oct Centre d'éducation des adultes de LaSalle (prise de photo à l'édifice Clément) 24-sept Centre d'éducation aux adultes Ferland 26-sept Centre d'éducation des adultes Jeanne-Sauvé 18-sept Centre François-Michelle (prise de photo au Collège Français) 15-oct Centre Gédéon-Ouimet 10-oct Centre Mountainview (prise de photo à John Grant High School) 29-sept Collège Stanislas 26-août Collège Ahuntsic 05-sept Collège André-Grasset 10-sept Collège Beaubois 18-sept Collège Bois-de-Boulogne 04-sept Collège Charlemagne 09-sept Collège d’Anjou 22-août Collège de Maisonneuve 28-29 août Collège de Montréal 03-sept Collège de Rosemont 07-oct Collège Français 15-oct Collège international des Marcellines (prise de photo à Villa Sainte-Marcelline) 30-sept Collège international Marie de France 14-oct Collège Jean-de-Brébeuf (Collégial) 15-sept Collège Jean-de-Brébeuf (Secondaire) 15-sept -

2015 GMAA Basketball Results
SPORTS LCC defence paves way to GMAA Bantam crown LCC 27 | ECS 14 By Mike Hickey The Suburban The Lower Canada College Lions employed an aggressive team defence en route to a con- vincing 27-14 win over Miss Edgar’s and Miss Cramp’s School to capture the GMAA Bantam Girls Championship Wednesday at Howard S. Billings High School. The two teams entered the title game evenly matched. They split a pair of regular season games, LCC beat ECS 18-11 in December and ECS won 29-26 in January, and were the top-seeded teams in the playoffs. LCC advanced to the finals when they defeated Howard S. MATT HICKEY / THE SUBURBAN Billings 50-6 while ECS downed “We prepared well defensively for this game,” LCC head coach Michael Moody said after the game. “We wanted to focus on 16 (Megan Bernier) and Royal West Academy 37-26 in 18 (Christiana Iannotti) to make it harder for them to score. Our guards did a god job of controlling the ball on offence.” semifinal action. LCC’s Amber Starke scored eight of her team’s first 10 points Bernier) and 18 (Christiana Ian- game’s Most Valuable Player and MVP performance, insisted that defence and it was a team victory.” as the Lions jumped out to a 10-2 notti) to make it harder for them earned praise from her coach. the championship was a team ac- Despite the loss ECS head coach lead which they would maintain to score. Our guards did a god job “Kaylee has been our leader all complishment. -

A Word from the Administration 8:30–8:50 AM Homeroom 8:53–9:18 AM Period 1 Mr
WWW.ROYALWESTACADEMY.COM MAY 2015 A Word From The Administration 8:30–8:50 AM Homeroom 8:53–9:18 AM Period 1 Mr. Tony Pita, Principal AM Mme Nathalie Cheff, Vice-Principal 9:21–9:46 Period 2 Ms. Chantal Juhasz, Vice-Principal 9:49–10:14 AM Period 3 10:14–10:24 AM Recess 10:24–10:49 AM Period 4 10:52–11:17 AM Period 5 11:17 AM–12:30 PM Lunch Students in Secondary I–IV are expected to empty their lockers by June 4, 2015, at which time locks must be returned to their homeroom teacher. Secondary V students are expected to have their lockers emptied and locks returned to homeroom teachers by June 3. It’s hard to believe but another amazing school year has Any unreturned locks will incur a cost of $8.50, which come and is almost done! Royal West Academy must be paid prior to receiving June report cards. students, parents, and staff, have all contributed to Please note that the cafeteria closed on May 29. The making the 2014–2015 school year a truly unforgettable regular exam schedule begins on June 8 and runs experience. through to June 19. Students need only report to Students and staff are hard at work preparing for end- school if an exam is scheduled. of-year exams. Parents should review the exam In early May, the administration distributed schedule carefully with their child. As per Ministry transportation allowance cheques to students. Only regulations, anyone who arrives more than 30 minutes students that live in the territory of the EMSB and at late for an exam will not be permitted to write the least 2.4 km from Royal West Academy are eligible for exam—thus receiving an unjustified absence. -

Knowing the Impact of Your Time on Your Community!
KNOWING THE IMPACT OF YOUR TIME ON YOUR COMMUNITY! “Some people only see volunteering as just helping someone or some people, but I see it as something different: changing and helping the world grow and strengthening your community and fulfilling your life.” ~Julia Falvo, Beaconsfield High School Gr8 Annual Report 2017-2018 MeSSaGe FroM tHe PreSident On behalf of the Board, we thank you for your support and VWI recruits and is blessed with passionate volunteers. With their presence here today. Our vision at Volunteer West Island is to help we assist non-profift organizations and community groups; continue to GROW volunteerism in our community. provide and support services such as Caring Paws Animal Therapy, the Income Tax Assistance Preparation, Contact, and Handyperson One particular focus this year has been our Meals on Wheels programs; and organize the Young at Heart 55+ Club and the Seniors program. MOW prepares and delivers 819 meals per week - a total Café. of 42, 753 meals per year. Our volunteers are amazing; many have been part of MOW for years, even decades. We have launched the Volunteer West Island has something for everybody! Let’s spread Meals on Wheels “Let’s Do Lunch” Campaign to attract additional the word: in our world of diminishing governmental assistance volunteers because we will be opening new MOW kitchens in a and reduced resources, volunteerism is fulfiflling and essential. proactive response to the growing need for this crucial service. Individual, Youth, and of course Corporate programs make all the difference in our community! Volunteer West Island would not exist if it were not for the volunteers and dedicated staff. -
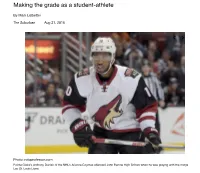
Making the Grade As a Student-Athlete | Education | Thesuburban.Com
9/1/2016 Making the grade as a student-athlete | Education | thesuburban.com http://www.thesuburban.com/life/education/making-the-grade-as-a-student-athlete/article_b2624573-4f67-5868- bec4-9dbfbdd7bdf8.html PREVIOUS Teachers are a special breed Making the grade as a student-athlete By Mark Lidbetter The Suburban Aug 31, 2016 Photo: rotoprofessor.com Pointe Claire’s Anthony Duclair of the NHL’s Arizona Coyotes attended John Rennie High School when he was playing with the midget AAA Lac St. Louis Lions. http://www.thesuburban.com/life/education/making-the-grade-as-a-student-athlete/article_b2624573-4f67-5868-bec4-9dbfbdd7bdf8.html 1/3 9/1/2016 Making the grade as a student-athlete | Education | thesuburban.com The term student-athlete is one that has become synonymous with Sport-études programs offered throughout Quebec. There are more than 450 programs active in the province that are geared to youth who have athletic and academic abilities and are recognized by their sporting federation. Local boards, such as the Lester B. Pearson and the English Montreal School Board offer Sport- Études through schools like Lester B. Pearson High School in St. Leonard and Pointe Claire’s John Rennie High School, respectively. The program covers a wide range of sports in team and individual disciplines as the academic side is the constant, while the sport can differ. The participants need to be categorized as an ‘Espoir’, ‘Relève, ‘Élite’ or ‘Excellence’ level athlete. Taking part in Sport-Études is no easy task but there are methods in place to help the student- athlete succeed on both the academic and athletic side of the equation. -

Results and Graduation Rates
RESULTS ON THE JUNE 2000 UNIFORM MINISTRY EXAMINATIONS BY SCHOOL BOARD AND BY EDUCATIONAL INSTITUTION FOR THE PUBLIC AND PRIVATE SCHOOL SYSTEMS AND GRADUATION RATES BY SCHOOL BOARD RESULTS ON THE JUNE 2000 UNIFORM MINISTRY EXAMINATIONS BY SCHOOL BOARD AND BY EDUCATIONAL INSTITUTION FOR THE PUBLIC AND PRIVATE SCHOOL SYSTEMS AND GRADUATION RATES BY SCHOOL BOARD Ministère de l'Éducation Direction de la sanction des études This report has been prepared by the Direction de la sanction des études with the cooperation of the Direction des statistiques et des études quantitatives of the Secteur de la planification. The English version has been translated by the Direction de la production en langue anglaise of the Services à la communauté anglophone. © Gouvernement du Québec Ministère de l'Éducation, 2001 ISBN 2-550-37251-4 Legal deposit–Bibliothèque nationale du Québec, 2001 TABLE OF CONTENTS INTRODUCTION...................................................................................................................... 1 PART I 1. ORGANIZATION OF THE EXAMINATION SESSION...............................................5 1.1 Coordination .........................................................................................................5 1.2 Certification Requirements ...................................................................................5 1.3 Preparation of Uniform Examinations..................................................................6 1.4 Administration of Examinations...........................................................................6 -

Results and Graduation Rates
Couvert 99_1062 A_PDF 4/13/00 4:30 PM Page 1 RESULTS ON THE JUNE 1999 UNIFORM MINISTRY EXAMINATIONS BY SCHOOL BOARD AND BY EDUCATIONAL INSTITUTION FOR THE PUBLIC AND PRIVATE SCHOOL SYSTEMS AND GRADUATION RATES BY SCHOOL BOARD Gouvernement du Québec Ministère de l’Éducation 16-7407-60A RESULTS ON THE JUNE 1999 UNIFORM MINISTRY EXAMINATIONS BY SCHOOL BOARD AND BY EDUCATIONAL INSTITUTION FOR THE PUBLIC AND PRIVATE SCHOOL SYSTEMS AND GRADUATION RATES BY SCHOOL BOARD Ministère de l’Éducation Direction de la sanction des études This report has been prepared by the Direction de la sanction des études with the cooperation of the Direction des statistiques et des études quantitatives of the Direction générale des services à la gestion. The English version has been translated by the Direction de la production en langue anglaise of the Services à la communauté anglophone. © Gouvernement du Québec Ministère de l'Éducation, 2000 ISBN 2-550-35630-6 Legal Deposit–Bibliothèque nationale du Québec, 2000 TABLE OF CONTENTS LIST OF TABLES INTRODUCTION................................................................................................................. 1 PART I 1. ORGANIZATION OF THE EXAMINATION SESSION ........................................... 5 1.1 Coordination .................................................................................................... 5 1.2 Certification Requirements............................................................................... 5 1.3 Preparation of Uniform Examinations ............................................................. -

Learning in the NEXT DECADE of EDUCATION Celebrating 15 Years!
LEArNING IN THE NEXT DECADE OF EDUCATION Celebrating 15 years! ANNUAL REPORT 2019-2020 table of 4 About Us 5 LEARN's structure contents 6 Learn’s Provincial Network 7 Message from the CEO 8 Message from the Chairman 10 Pedagogical Services Team 38 Virtual Campus Team 52 Provincial Resource Team 64 Thank you e AAboutbout uuss LEARN is a non-profit educational organization that offers, at no charge, a wealth of information and resources for the English-speaking community in Quebec. Building on the merger of three organizations in July 2005, LEARN has developed a variety of resources. LEARN is a driving force for educational innovation and practice as well as an internationally recognized leader in the field on e-learning. The learn team is composed of highly skilled and experienced educators and professionals who bring to the community a vast array of resources and services which are coupled with a collaborative and success-oriented mindset. At the heart of the organization, and its distinguishing feature, is a deep-rooted passion for education. LEARN recognizes that learning does begin and end in a school and that learning is a lifelong endeavor. To contribute to this process of lifelong learning, learn works closely with its educational and community partners and in harmony with the province’s curriculum, and hosts resources that are not only core to the curriculum but which both complement and supplement it. Learn’s focus is consistently on making learning engaging, effective, and ongoing. Vision mission values To be the most innovative To serve the educational Expertise and collaborative lifelong learning needs Knowledge building educational organization of the English-speaking Collaboration community in Quebec for the English-speaking Innovation community of Quebec. -

Enhanced Student Information System (ESIS) ESIS Data Dictionary
Enhanced Student Information System (ESIS) ESIS Data Dictionary First Edition How to obtain more information Specific inquiries about this product and related statistics or services should be directed to: Client Services, Culture, Tourism and the Centre for Education Statistics, Statistics Canada, Ottawa, Ontario, K1A 0T6 (telephone: (613) 951-7608; toll free at 1 800 307-3382; by fax at (613) 951-9040; or e-mail: [email protected]). For information on the wide range of data available from Statistics Canada, you can contact us by calling one of our toll-free numbers. You can also contact us by e-mail or by visiting our Web site. National inquiries line 1 800 263-1136 National telecommunications device for the hearing impaired 1 800 363-7629 E-mail inquiries [email protected] Web site www.statcan.ca Ordering information This product, is available on the Internet for free. Users can obtain single issues at: http://www.statcan.ca/english/sdds/5017.htm Standards of service to the public Statistics Canada is committed to serving its clients in a prompt, reliable and courteous manner and in the official language of their choice. To this end, the Agency has developed standards of service which its employees observe in serving its clients. To obtain a copy of these service standards, please contact Statistics Canada toll free at 1 800 263-1136. Enhanced Student Information System (ESIS) ESIS Data Dictionary Note of appreciation Canada owes the success of its statistical system to a long-standing partnership between Statistics Canada, the citizens of Canada, its businesses, governments and other institutions. -

Classement Des Écoles Secondaires Québécoises Par Fraser En 2004
Classement des écoles Notes importantes à propos pour toutes les années visées. des classements Les écoles secondaires de la province ne figurent pas toutes aux tableaux ni au classement. En sont exclues Dans ce tableau, les écoles sont classées (du côté gauche les écoles où l’on comptait moins de 15 élèves de 5e se- de la page) en ordre décroissant (de 1 à 457) selon leur condaire. Les séries de données disponibles sur ces performance scolaire telle que mesurée d’après la cote écoles n’étaient pas suffisantes pour que l’on puisse en globale sur 10 (figurant du côté droit du tableau) pour faire une analyse juste et raisonnable. l’année scolaire 2002-2003. Sont également énumérées Sont également exclus les centres d’éducation pour les statistiques moyennes de classement et de cote glo- adultes, les services d’éducation permanente, les insti- bale de l’école pour l’ensemble des cinq dernières tutions qui reçoivent essentiellement des élèves années. Plus la cote globale sur 10 d’une école est élevée, étrangers non résidents, les écoles dont les données plus elle se rapproche des premiers rangs. Les écoles qui étaient insuffisantes et les établissements alternatifs. ont obtenu des cotes globales identiques ont reçu le L’exclusion d’une école du Bulletin ne devrait même rang. La mention «nd» figure au tableau dans nullement être considérée comme l’expression d’un les cas où l’on n’a pas pu obtenir des données historiques jugement sur l’efficacité de cet établissement. ––––– Rang ––––– Cote globale 2002/ 1999/ Région Secteur 2002/ 1999/ 2003 -

Honorary Chairperson
HONORARY CHAIRPERSON The Loyola-Ed Meagher Sports Tournament Committee is honoured to introduce Wes Sanders as Honou- rary Chairman for this year’s 49th annual tournament. Wes arrived at Loyola in the wake of a tragedy. On June 23,1985, the day after Loyola’s convocation ceremo- ny, Loyola lost its beloved chemistry and physics teacher Nish Mukerji in the terrorist bombing of Air India Flight 182. Dr. Mukerji, along with his wife Shefali, were returning to their native India to visit friends and family. All 329 people on board the flight were killed when the plane exploded off the coast of Ireland. While the impact of Dr. Mukerji’s loss on the Loyola community was immeasurable, Loyola was fortunate that a young and talented Wesley Sanders was hired to address the massive void left in the school’s senior science department. Now, thirty-four years later, Wes himself has assumed the role as “senior statesman” and is currently the school’s longest serving faculty member. He has been, and continues to be, an integral part of Loyola High School, having served admirably as both educator and coach. Wes has used his engineering background to become a master teacher. While he continues to teach phys- ics to Loyola’s aspiring scientists, as well as the school’s demanding honours level mathematics courses, he broadened his teaching repertoire a number of years ago by offering to teach a Secondary Five option course, Culinary Arts. Wes now shares his talents and passion for cooking with a group of aspiring student chefs. With a teaching style that can best be described as professional and fair, Wes also has the unique ability to bring humour into his classes. -

West Island Seniors' Index
The West Island Community Resource Centre would like to thank the West Island SENIORS Resource Guide following community partners for giving their input into the creation of this guide: 8th Edition Arthritis West Island Self-Help (AWISH) DDO Seniors Club Published by: the West Island Community Resource Centre (CRC) Gerontologis SPVM Police Station 3 NOVA West Island Pointe-Claire Aid for Seniors West Island Citizen Advocacy (WICA) West Island Association for Intellectually Handicapped (WIAIH) CENTRE DE RESsOURCES COMMUNAUTAIREs DE L'OUEST-DE-L'ILE Volunteer West Island West Island Community Resource Centre Thank you to our special team of volunteers who dedicated their time and efforts to making this guide possible: The West Island Seniors Resource Guide is a source of information Joanne, Ruth and Eliane for seniors, their families and caregivers, and professionals who are seeking support services and programs for elderly people in the West Island community. We would also like to thank all of our funding partners for supporting the West Island CRC. Disclaimer: The West Island Community Resource Centre (CRC) provides these resources as information only. It is not intended to provide medical, legal or professional advice whatsoever and should A special thank you to: not be relied upon in that respect. Inclusion of an organization and Centraide of Greater Montreal information about its programs and services on the Guide does not imply endorsement by the CRC nor does exclusion indicate lack West Island Community Shares of endorsement. The CRC makes no representations or warranties City of Pointe-Claire express or implied regarding the products or services of the resources listed or advertised in the Guide.