How Much Longer Will It Take?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Modelling the Distribution of Alfonsino, Beryx Splendens, Over The
Abstract.- Commercial and Modelling the distribution of scientificbottom longline catches of alfonsino, Beryx splendens, from alfonsino, Beryx splendens, over seamounts off New Caledonia were sampled to study length-frequency distributions. A total of 14,674 fish the seamounts of New Caledonia were measured. CPUE of Beryx splendens on two seamounts is mod- Patrick Lehodey elled in terms of length and depth. The data show that mean length in- Paul Marchal creases with depth; this is well de- scribed by a bivariate normal model Rene Grandperrin that estimates catch for a given sea- Centre ORSTOM, BP A5, Noumea, New Caledonia mount. In addition, the data show I that mean length also varies with the depth of the top of seamounts; this is described by a recursive model that is designed to predict ap- proximate catch for any seamount. A bottom longline fishery operated recursive model predicts catch on The limitations of both models are on the seamounts of the Exclusive any seamount. discussed, particularly with regard Economic Zone (EEZ) of New Cale- to temporal variation. donia from February 1988 to July 1991.l Three vessels were involved Material and methods but only one vessel was operated at any given time. The fishing effort, Data which totalled 4,691,635 hooks, fo- Alfonsino were captured with long- cused on five seamounts (B, C, D, line gear (Fig. 2). The main line, J, and K) whose summits are lo- averaging 4,000 m, was held on the cated at depths ranging from 500 1). bottom by means of terminal an- to 750 m (Fig. -
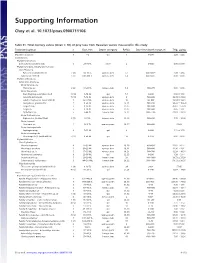
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Beryx Splendens Lowe, 1834
Beryx splendens Lowe, 1834 AphiaID: 126395 SPLENDID ALFONSINO Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Beryciformes (Ordem) > Berycidae (Familia) Heessen, Henk Sinónimos Bryx splendens Lowe, 1834 Referências MARTINS, R.; CARNEIRO, M., 2018. Manual de identificação de peixes ósseos da costa continental portuguesa – Principais Características Diagnosticantes. IPMA, I.P., 204p Fernandes, P., Collette, B., Heessen, H., Smith-Vaniz, W.F. & Herrera, J. 2015. Beryx splendens. The IUCN Red List of Threatened Species 2015: e.T16425354A45791585. Downloaded on 05 August 2019. additional source Froese, R. & D. Pauly (Editors). (2018). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; 1 Eschmeyer, W.N.; Froese, R. (2001). Pisces, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 357-374 [details] additional source Gulf of Maine Biogeographic Information System (GMBIS) Electronic Atlas. 2002. November, 2002. [details] additional source Hareide, N.R. and G. Garnes. 1998. The distribution and abundance of deep water fish along the Mid-Atlantic Ridge from 43°N to 61°N. Theme session on deep water fish and fisheries. ICES CM 1998/O:39. [details] additional source Welshman, D., S. Kohler, J. Black and L. Van Guelpen. 2003. An atlas of distributions of Canadian Atlantic fishes. , available online at http://epe.lac-bac.gc.ca/100/205/301/ic/cdc/FishAtlas/default.htm [details] additional source Streftaris, N.; Zenetos, A.; Papathanassiou, E. -

Alfonsino Species Sheet
ALFONSINO Beryx splendens Alfonsino have a firm white flesh with high oil content. Alfonsino is suitable for most cooking methods. Wild caught Alfonsino from New Zealand are caught all year round, at a depth of 200-800m. All Sealord Alfonsino are from sustainable and well managed fisheries. AVERAGE LENGTH WEIGHT AVAILABILITY CATCH METHOD 30-50 cm 1-1.5 kg All year round Trawl 11.8 – 19.7 inches 2.2 – 3.3 lbs SEALORD PROVIDE A RANGE OF PRODUCTS IN FROZEN FORMATS Format Description Size Grading Whole Whole fish Run of Catch 0.2-0.3kg / 0.3-0.5kg / 0.5-0.7kg / Dressed Headed, gutted, tail remains 0.7-1kg / over 1kg / Run of Catch SUSTAINABLE DEEPWATER SEAFOOD We care about the future of fishing, so our fish comes from well-managed fisheries, some of which have a Fisheries Improvement Plan in place. We continue to evolve our processes and undertake research to ensure we manage fisheries with the best practices and quality of scientific information available. The Quota Management System (QMS) has been operating in New Zealand for over thirty years, solidifying New Zealand’s reputation as a world leader in sustainable fisheries management. It ensures that our fisheries resources are not over-fished and that our seafood will be available for generations to come. It is one of the most extensive quota-based fisheries management systems in the world, with over 100 species or species complexes managed within this framework. CATCH AREA NUTRITION BYX 10 Energy 597kJ Protein 18.4g BYX 1 Fat Total 7.6g Saturated 2.0g Carbohydrate 0.2g Sugars 0.2g BYX 8 Sodium 35mg BYX 7 BYX 2 OUR ACCREDITATIONS BYX 3 INCLUDE: TIFIED ER BYX 3 C ACCP H Y T FO E OD SAF. -

LESSON 2 DNA Barcoding and the Barcode of Life Database
LESSON 2 DNA Barcoding 2 and the Barcode of Life Database (BOLD) Introduction In this lesson, students will receive an “unknown” DNA sequence and use the bioinformatics tool Basic Local Alignment Search Tool (BLAST) to identify the species from which the sequence came. Students then visit the Barcode of Life Database (BOLD) to obtain taxonomic information about their species and form taxonomic groups for scientific collaboration. The lesson ends with each student generating a hypothesis about the relatedness of the species within each group. In Lesson Two, students also learn how postdoctoral scientists in DNA and history might use bioinformatics tools in their career. Learning Objectives Class Time At the end of this lesson, students will know that: 1 class period of 50 minutes if Student • Bioinformatics tools are used by people in many different career fields, Reading is assigned as homework; including postdoctoral scientists in DNA and history. otherwise 2 class periods of 50 • Bioinformatics tools such as BLAST and databases like the National Center for minutes each. Biotechnology Information (NCBI) and the Barcode of Life Database (BOLD) can be used to identify unknown DNA sequences and obtain information Prior Knowledge Needed about the species from which the sequences came. • DNA contains the genetic information • Scientific names for species, including the genus and species names, can be that encodes traits. used to classify species based on evolutionary relatedness. • Basic knowledge of taxonomy (specifically the different categories • Scientists from around the world compile their data into databases such as used in taxonomy to classify those at the NCBI and BOLD to encourage scientific collaboration and increase organisms, and that the study scientific knowledge. -

Historical Extent of Decline
NMFS / Interagency Working Group Evaluation of CITES Criteria and Guidelines Pamela M. Mace (Chair) Andy W. Bruckner Nancy K. Daves John D. Field John R. Hunter Nancy E. Kohler Robert G. Kope Susan S. Lieberman Margaret W. Miller James W. Orr Robert S. Otto Tim D. Smith Nancy B. Thompson with contributions from Julie Lyke and Arthur G. Blundell U.S. Department of Commerce National Oceanic and Atmospheric Administration National Marine Fisheries Service NOAA Technical Memorandum NMFS-F/SPO-58 October 2002 -1- NMFS / Interagency Working Group Evaluation of CITES Criteria and Guidelines Pamela M. Mace (Chair) Andy W. Bruckner Nancy K. Daves John D. Field John R. Hunter Nancy E. Kohler Robert G. Kope Susan S. Lieberman Margaret W. Miller James W. Orr Robert S. Otto Tim D. Smith Nancy B. Thompson with contributions from Julie Lyke and Arthur G. Blundell NOAA Technical Memorandum NMFS-F/SPO-58 October 2002 U.S. Department of Commerce Donald L. Evans, Secretary National Oceanic and Atmospheric Administration Vice Admiral Conrad C. Lautenbacher, Jr., USN (Ret.) Under Secretary for Oceans and Atmosphere National Marine Fisheries Service William T. Hogarth, Assistant Administrator for Fisheries -2- This document is the result of several meetings and teleconferences of the NMFS / Interagency Working Group to evaluate CITES criteria and guidelines, held over a two-year period beginning in October 2000. The purposes were to evaluate existing CITES criteria and guidelines, to suggest improvements, and to evaluate the pro- posed improvements for a variety of marine and other taxa. Suggested citation: Mace, P.M., A.W. Bruckner, N.K. -

Alfonsino (Byx)
ALFONSINO (BYX) ALFONSINO (BYX) (Beryx splendens, B. decadactylus) BYS BYD 1. FISHERY SUMMARY Alfonsino was introduced into the Quota Management System (QMS) on 1 October 1986, with allowances, TACCs and TACs in Table 1. Table 1: Recreational and Customary non-commercial allowances, TACCs and TACs for alfonsino by Fishstock. Fishstock Recreational Allowance Customary non-commercial TACC TAC allowance BYX 1 2 2 300 304 BYX 2 - - 1 575 1 575 BYX 3 - - 1 010 1 010 BYX 7 - - 80.5 80.5 BYX 8 - - 20 20 BYX 10 - - 10 10 1.1 Commercial fisheries Alfonsino has supported a major mid-water target trawl fishery off the east coast of the North Island since 1983 and is a minor bycatch of other trawl fisheries around New Zealand. The original gazetted TACs were based on the 1983–84 landings except for BYX 10 which was administratively set. Recent reported domestic landings and actual TACCs are shown in Table 1, while Figure 1 shows the historical landings and TACC values for the main BYX stocks. Alfonsino landings in New Zealand consist almost entirely of one species, Beryx splendens: the other species, B. decadactylus, is thought to make up less than 1% of landings. Before 1983 alfonsino were virtually unfished, but two main fisheries now exist in New Zealand. The first to develop was the lower east coast North Island fishery (BYX 2), which developed in the mid-1980s. The other is the eastern Chatham Rise fishery (BYX 3), which developed in the mid-1990s. Alfonsino are caught throughout the New Zealand EEZ but only in small quantities outside of the east coast North Island and eastern Chatham Rise fisheries. -

Application of Surplus-Production Models to Splendid Alfonsin Stock in the Southern Emperor and Northern Hawaiian Ridge (SE-NHR)
Appendix C Application of Surplus-Production Models to Splendid Alfonsin Stock in the Southern Emperor and Northern Hawaiian Ridge (SE-NHR) Akira NISHIMURA1 and Akihiko YATSU 2 1 Hokkaido National Fisheries Research Institute, Fisheries Research Agency, Japan 2 Seikai National Fisheries Research Institute, Fisheries Research Agency, Japan The Japanese trawlers commenced exploratory fishing operations in the Southern Emperor and Northern Hawaiian Ridge (SE-NHR) in 1969, and the trawl fishery have been developed after then. In this area, 2 to 13 trawlers have been conducting fishing activities every year, targeting mainly North Pacific armorhead (Pseudopentaceros wheeleri) and splendid alfonsino (Beryx splendens). The fisheries are the important component of the ecosystem in the fishing ground, and understanding the impacts of the fisheries is important for the sustainable use of the aquatic resources. To estimate the management benchmarks, first attempt has been made to apply surplus production model to the splendid alfonsino stock in SE-NHR (Nishimura & Yatsu, 2006). This paper describe the results from two different surplus-production model program runs with using unadjusted/adjusted CPUE, and with using catch statistics from Japan, Korea, and USSR/Russia. 1. Catch and CPUE data Japanese fishery statistics in SE-NHR were complied by the Far Seas Fisheries Research Institute (Shimizu) and Hokkaido National Fisheries Research Institute (Kushiro) based on operation records submitted to the Fisheries Agency of Japan from commercial fishing vessels (Yanagimoto and Nishimura, 2007). Korea and USSR/Russia catch data of alfonsino was also prepared by Korea and Russian Institutes. These data sets of alfonsino were submitted to the Science Working Group of NWPBT meetings, and these data sets were combined and used for the following analyses.