Historical Extent of Decline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -

1 a Petition to List the Oceanic Whitetip Shark
A Petition to List the Oceanic Whitetip Shark (Carcharhinus longimanus) as an Endangered, or Alternatively as a Threatened, Species Pursuant to the Endangered Species Act and for the Concurrent Designation of Critical Habitat Oceanic whitetip shark (used with permission from Andy Murch/Elasmodiver.com). Submitted to the U.S. Secretary of Commerce acting through the National Oceanic and Atmospheric Administration and the National Marine Fisheries Service September 21, 2015 By: Defenders of Wildlife1 535 16th Street, Suite 310 Denver, CO 80202 Phone: (720) 943-0471 (720) 942-0457 [email protected] [email protected] 1 Defenders of Wildlife would like to thank Courtney McVean, a law student at the University of Denver, Sturm college of Law, for her substantial research and work preparing this Petition. 1 TABLE OF CONTENTS I. INTRODUCTION ............................................................................................................................... 4 II. GOVERNING PROVISIONS OF THE ENDANGERED SPECIES ACT ............................................. 5 A. Species and Distinct Population Segments ....................................................................... 5 B. Significant Portion of the Species’ Range ......................................................................... 6 C. Listing Factors ....................................................................................................................... 7 D. 90-Day and 12-Month Findings ........................................................................................ -

How Much Longer Will It Take?
How much longer will it take? A ten-year review of the implementation of United Nations General Assembly resolutions 61/105, 64/72 and 66/68 on the management of bottom fisheries in areas beyond national jurisdiction FULL REPORT – AUGUST 2016 DAVID SHALE/NATURE PICTURE LIBRARAY SHALE/NATURE DAVID Leiopathes sp., a deepwater black coral, has lifespans in excess of 4,200 years (Roark et al., 2009*), making it one of the oldest living organism on Earth. Specimen was located off the coast of Oahu, Hawaii, in ~400 m water depth. * Roark, E.B., Guilderson, T.P., Dunbar, R.B., Fallon, S.J., and Mucciarone, D.A., 2009. Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences, 106: 520– 5208, doi: 10.1073/pnas.0810875106. © HAWAII UNDERSEA RESEARCH LABORATORY, TERRY KERBY AND MAXIMILIAN CREMER Contents Executive summary 03 1.0 Introduction 09 2.0 North Atlantic 10 2.1 Northeast Atlantic 10 2..2 Northwest Atlantic 21 3.0 South Atlantic 33 3.1 Southeast Atlantic 33 3.2 Southwest Atlantic and other non-RFMO areas 39 4.0 North Pacific 42 Citation: 5.0 South Pacific 49 Gianni, M., Fuller, S.D., Currie, D.E.J., Schleit, 6.0 Indian Ocean 60 K., Goldsworthy, L., Pike, B., Weeber, B., 7.0 Southern Ocean 66 Owen, S., Friedman, A. How much longer will it take? A ten-year 8.0. Mediterranean Sea 71 review of the implementation of United Annex 1. Acronyms 73 Nations General Assembly resolutions Annex 2. History of the UNGA negotiations 73 61/105, 64/72 and 66/68 on the management Annex 3. -

Empire and Architecture at 16Th-Century Puerto Real, Hispaniola: an Archeological Perspective
EMPIRE AND ARCHITECTURE AT 16th -CENTURY PUERTO REAL, HISPANIOLA: AN ARCHEOLOGICAL PERSPECTIVE BY RAYMOND F. WILLIS DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1984 Copyright 1984 by Raymond F. Willis t; ACKNOWLEDGEMENTS The research for this dissertation was funded by the Organization of American States, University of Florida, and Wentworth Foundation. I would first like to thank four individuals whose support and guidance made possible the accomplishment of successful, large-scale archeological research in Haiti: Albert Mangones, director of Haiti's Institute de Sauvegarde du Patrimoine National (ISPAN) ; Ragnar Arnesen, director of Haiti's Organization of American States (OAS) delegation; Dr. William Hodges, director of the Hopital le Bon Samaritain at Limbe , Haiti; and, Paul Hodges, a good friend and the best site manager and most competent field photographer I will ever have the pleasure to work with. Without the guidance of these four men I could not have come near to accomplishing my goals; all four have the deepest concern for the welfare and upward progression of the Haitian people. I can only hope that this dissertation will illustrate that the support and trust they gave me were not wasted. Many individuals at the University of Florida aided me and supported this project over the past six years. First is my original committee chairman (now retired) Dr. Charles H. Fairbanks, Distinguished Service iii . Professor m Anthropology. Dr. Fairbanks was the original principal investigator and prime mover for the initiation of the Puerto Real project. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Sharks in the Seas Around Us: How the Sea Around Us Project Is Working to Shape Our Collective Understanding of Global Shark Fisheries
Sharks in the seas around us: How the Sea Around Us Project is working to shape our collective understanding of global shark fisheries Leah Biery1*, Maria Lourdes D. Palomares1, Lyne Morissette2, William Cheung1, Reg Watson1, Sarah Harper1, Jennifer Jacquet1, Dirk Zeller1, Daniel Pauly1 1Sea Around Us Project, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada 2UNESCO Chair in Integrated Analysis of Marine Systems. Université du Québec à Rimouski, Institut des sciences de la mer; 310, Allée des Ursulines, C.P. 3300, Rimouski, QC, G5L 3A1, Canada Report prepared for The Pew Charitable Trusts by the Sea Around Us project December 9, 2011 *Corresponding author: [email protected] Sharks in the seas around us Table of Contents FOREWORD........................................................................................................................................ 3 EXECUTIVE SUMMARY ................................................................................................................. 5 INTRODUCTION ............................................................................................................................... 7 SHARK BIODIVERSITY IS THREATENED ............................................................................. 10 SHARK-RELATED LEGISLATION ............................................................................................. 13 SHARK FIN TO BODY WEIGHT RATIOS ................................................................................ 14 -

Fish Assemblages of Caribbean Coral Reefs: Effects of Overfishing on Coral Communities Under Climate Change
FISH ASSEMBLAGES OF CARIBBEAN CORAL REEFS: EFFECTS OF OVERFISHING ON CORAL COMMUNITIES UNDER CLIMATE CHANGE Abel Valdivia-Acosta A Dissertation Submitted to the Faculty of University of North Carolina at Chapel Hill In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biological Sciences in the Department of Biology, College of Art and Sciences. Chapel Hill 2014 Approved by: John Bruno Charles Peterson Allen Hurlbert Julia Baum Craig Layman © 2014 Abel Valdivia-Acosta ALL RIGHTS RESERVED ii ABSTRACT Abel Valdivia-Acosta: Fish assemblages of Caribbean coral reefs: Effects of overfishing on coral communities under climate change (Under the direction of John Bruno) Coral reefs are threatened worldwide due to local stressors such as overfishing, pollution, and diseases outbreaks, as well as global impacts such as ocean warming. The persistence of this ecosystem will depend, in part, on addressing local impacts since humanity is failing to control climate change. However, we need a better understanding of how protection from local stressors decreases the susceptibility of reef corals to the effects of climate change across large-spatial scales. My dissertation research evaluates the effects of overfishing on coral reefs under local and global impacts to determine changes in ecological processes across geographical scales. First, as large predatory reef fishes have drastically declined due to fishing, I reconstructed natural baselines of predatory reef fish biomass in the absence of human activities accounting for environmental variability across Caribbean reefs. I found that baselines were variable and site specific; but that contemporary predatory fish biomass was 80-95% lower than the potential carrying capacity of most reef areas, even within marine reserves. -

Modelling the Distribution of Alfonsino, Beryx Splendens, Over The
Abstract.- Commercial and Modelling the distribution of scientificbottom longline catches of alfonsino, Beryx splendens, from alfonsino, Beryx splendens, over seamounts off New Caledonia were sampled to study length-frequency distributions. A total of 14,674 fish the seamounts of New Caledonia were measured. CPUE of Beryx splendens on two seamounts is mod- Patrick Lehodey elled in terms of length and depth. The data show that mean length in- Paul Marchal creases with depth; this is well de- scribed by a bivariate normal model Rene Grandperrin that estimates catch for a given sea- Centre ORSTOM, BP A5, Noumea, New Caledonia mount. In addition, the data show I that mean length also varies with the depth of the top of seamounts; this is described by a recursive model that is designed to predict ap- proximate catch for any seamount. A bottom longline fishery operated recursive model predicts catch on The limitations of both models are on the seamounts of the Exclusive any seamount. discussed, particularly with regard Economic Zone (EEZ) of New Cale- to temporal variation. donia from February 1988 to July 1991.l Three vessels were involved Material and methods but only one vessel was operated at any given time. The fishing effort, Data which totalled 4,691,635 hooks, fo- Alfonsino were captured with long- cused on five seamounts (B, C, D, line gear (Fig. 2). The main line, J, and K) whose summits are lo- averaging 4,000 m, was held on the cated at depths ranging from 500 1). bottom by means of terminal an- to 750 m (Fig. -
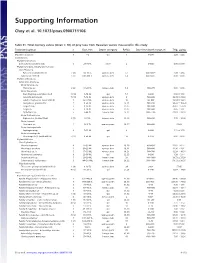
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Beryx Splendens Lowe, 1834
Beryx splendens Lowe, 1834 AphiaID: 126395 SPLENDID ALFONSINO Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Beryciformes (Ordem) > Berycidae (Familia) Heessen, Henk Sinónimos Bryx splendens Lowe, 1834 Referências MARTINS, R.; CARNEIRO, M., 2018. Manual de identificação de peixes ósseos da costa continental portuguesa – Principais Características Diagnosticantes. IPMA, I.P., 204p Fernandes, P., Collette, B., Heessen, H., Smith-Vaniz, W.F. & Herrera, J. 2015. Beryx splendens. The IUCN Red List of Threatened Species 2015: e.T16425354A45791585. Downloaded on 05 August 2019. additional source Froese, R. & D. Pauly (Editors). (2018). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; 1 Eschmeyer, W.N.; Froese, R. (2001). Pisces, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 357-374 [details] additional source Gulf of Maine Biogeographic Information System (GMBIS) Electronic Atlas. 2002. November, 2002. [details] additional source Hareide, N.R. and G. Garnes. 1998. The distribution and abundance of deep water fish along the Mid-Atlantic Ridge from 43°N to 61°N. Theme session on deep water fish and fisheries. ICES CM 1998/O:39. [details] additional source Welshman, D., S. Kohler, J. Black and L. Van Guelpen. 2003. An atlas of distributions of Canadian Atlantic fishes. , available online at http://epe.lac-bac.gc.ca/100/205/301/ic/cdc/FishAtlas/default.htm [details] additional source Streftaris, N.; Zenetos, A.; Papathanassiou, E.