SEAFO SCR Doc 01/2009 SPECIES PROFILE PROPOSAL for THE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

PENTACEROTIDAE Pelagic Armorhead Distribution
PENTACEROTIDAE Pelagic Armorhead by Richard N. Uchida Valid name Pseudopentaceros wheeleri Hardy 1983 (Fig. 68) Synonymy Pseudopentaceros richardsoni Abe 1957 Pentaceros richardsoni Follett and Dempster 1963 Pseudopentaceros wheeferi Hardy 1983 (?) Pseudopentaceros pectoralis Hardy 1983 (from Hardy 1983) Common and vernacular names Pelagic armorhead; armorhead; boarfish; kusakari tsubodai Distribution The pelagic armorhead, unlike many mesopelagic seamount species, does not confine itself exclusively to the demersal environ- Occurs primarily at Hancock Seamounts in the NWHI at depths ment throughout its lifetime but also migrates into the epipelagic of 256-366 m; also at Kure Atoll and Ladd Seamount (its presence regime. Catches by Japanese trawlers indicate the fish at depths at these latter two locations representing a range extension). l9 between 300 and 600 m over the central North Pacific seamounts. Other reports indicate that pelagic armorhead is caught in salmon gill nets or with handline near the surface. The species has also Distinguishing characteristics been found in stomachs of surface feeding sei whales. This points to the possibility of extensive vertical migration by the species. D. XIII-XIV, 8-9; A. IV, 7-8; PI. 17-18; Gr. 7-8+16-18. Body Investigators disagree on time of vertical movement. Some report ovate and compressed; dorsal and anal fin profiles evenly curved; that pelagic armorhead rises to the surface at night, feeding on head pointed, encased in exposed striated bones, some of which organisms such as euphausids, mysids, copepods, salps, shrimps, are rugulose or finely wrinkled. Dorsal spines strong, heterocanth, and myctophids which are usually associated with the deep-scattering longitudinally ridged. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -
![Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East](https://docslib.b-cdn.net/cover/1518/stock-status-report-alfonsino-bys-doc-sc-11-2019-south-east-341518.webp)
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 South East
Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 STATUS REPORT Beryx splendens Alfonsino FAO -ASFIS code: BYS 2019 Updated 21 November 2019 South East Atlantic Fisheries Organization [SEAFO] 1 Stock Status Report: Alfonsino [BYS] DOC/SC/11/2019 TABLE OF CONTENTS 1. Description of the fishery ....................................................................................................................... 3 1.1 Description of fishing vessels and fishing gear .......................................................................... 3 1.2 Spatial and temporal distribution of fishing ............................................................................... 6 1.3 Reported retained catches and discards ..................................................................................... 9 1.4 IUU catch ............................................................................................................................... 12 2. Stock distribution and identity ............................................................................................................. 12 3. Data available for assessments, life history parameters and other population information ..................... 12 3.1 Fisheries and surveys data ....................................................................................................... 12 3.2 Length data and frequency distribution ................................................................................... 13 3.3 Length-weight relationships ................................................................................................... -

How Much Longer Will It Take?
How much longer will it take? A ten-year review of the implementation of United Nations General Assembly resolutions 61/105, 64/72 and 66/68 on the management of bottom fisheries in areas beyond national jurisdiction FULL REPORT – AUGUST 2016 DAVID SHALE/NATURE PICTURE LIBRARAY SHALE/NATURE DAVID Leiopathes sp., a deepwater black coral, has lifespans in excess of 4,200 years (Roark et al., 2009*), making it one of the oldest living organism on Earth. Specimen was located off the coast of Oahu, Hawaii, in ~400 m water depth. * Roark, E.B., Guilderson, T.P., Dunbar, R.B., Fallon, S.J., and Mucciarone, D.A., 2009. Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences, 106: 520– 5208, doi: 10.1073/pnas.0810875106. © HAWAII UNDERSEA RESEARCH LABORATORY, TERRY KERBY AND MAXIMILIAN CREMER Contents Executive summary 03 1.0 Introduction 09 2.0 North Atlantic 10 2.1 Northeast Atlantic 10 2..2 Northwest Atlantic 21 3.0 South Atlantic 33 3.1 Southeast Atlantic 33 3.2 Southwest Atlantic and other non-RFMO areas 39 4.0 North Pacific 42 Citation: 5.0 South Pacific 49 Gianni, M., Fuller, S.D., Currie, D.E.J., Schleit, 6.0 Indian Ocean 60 K., Goldsworthy, L., Pike, B., Weeber, B., 7.0 Southern Ocean 66 Owen, S., Friedman, A. How much longer will it take? A ten-year 8.0. Mediterranean Sea 71 review of the implementation of United Annex 1. Acronyms 73 Nations General Assembly resolutions Annex 2. History of the UNGA negotiations 73 61/105, 64/72 and 66/68 on the management Annex 3. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Modelling the Distribution of Alfonsino, Beryx Splendens, Over The
Abstract.- Commercial and Modelling the distribution of scientificbottom longline catches of alfonsino, Beryx splendens, from alfonsino, Beryx splendens, over seamounts off New Caledonia were sampled to study length-frequency distributions. A total of 14,674 fish the seamounts of New Caledonia were measured. CPUE of Beryx splendens on two seamounts is mod- Patrick Lehodey elled in terms of length and depth. The data show that mean length in- Paul Marchal creases with depth; this is well de- scribed by a bivariate normal model Rene Grandperrin that estimates catch for a given sea- Centre ORSTOM, BP A5, Noumea, New Caledonia mount. In addition, the data show I that mean length also varies with the depth of the top of seamounts; this is described by a recursive model that is designed to predict ap- proximate catch for any seamount. A bottom longline fishery operated recursive model predicts catch on The limitations of both models are on the seamounts of the Exclusive any seamount. discussed, particularly with regard Economic Zone (EEZ) of New Cale- to temporal variation. donia from February 1988 to July 1991.l Three vessels were involved Material and methods but only one vessel was operated at any given time. The fishing effort, Data which totalled 4,691,635 hooks, fo- Alfonsino were captured with long- cused on five seamounts (B, C, D, line gear (Fig. 2). The main line, J, and K) whose summits are lo- averaging 4,000 m, was held on the cated at depths ranging from 500 1). bottom by means of terminal an- to 750 m (Fig. -

New Records of Fishes from the Hawaiian Islands!
Pacific Science (1980), vol. 34, no. 3 © 1981 by The University Press of Hawaii. All rights reserved New Records of Fishes from the Hawaiian Islands! JOHN E. RANDALL 2 ABSTRACT: The following fishes represent new records for the Hawaiian Islands: the moray eel Lycodontis javanicus (Bleeker), the frogfish Antennarius nummifer (Cuvier), the jack Carangoides ferdau (Forssk::U), the grouper Cromileptes altivelis (Cuvier) (probably an aquarium release), the chubs Kyphosus cinerascens (Forsskal) and K. vaigiensis (Quoy and Gaimard), the armorhead Pentaceros richardsoni Smith, the goatfish Upeneus vittatus (Forsskal) (a probable unintentional introduction by the Division of Fish and Game, State of Hawaii), the wrasse Halichoeres marginatus Ruppell,' the gobies Nemateleotris magnifica Fowler and Discordipinna griessingeri Hoese and Fourmanoir, the angelfish Centropyge multicolor Randall and Wass, the surgeonfish Acanthurus lineatus (Linnaeus), the oceanic cutlassfish Assurger anzac (Alexander), and the driftfish Hyperoglyphe japonica (Doderlein). In addition, the snapper Pristipomoides auricilla (Jordan, Evermann, and Tanaka) and the wrasse Thalassoma quinquevittatum (Lay and Bennett), both overlooked in recent compilations, are shown to be valid species for the Hawaiian region. Following Parin (1967), the needlefish Tylosurus appendicu latus (Klunzinger), which has a ventral bladelike bony projection from the end of the lower jaw, is regarded as a morphological variant of T. acus (Lacepede). IN 1960, W. A. Gosline and V. E. Brock modified by Randall and Caldwell (1970). achieved the difficult task of bringing the fish Randall (1976) reviewed the additions to, fauna of the Hawaiian Islands into one com and alterations in, the nomenclature of the pact volume, their Handbook of Hawaiian Hawaiian fish fauna to 1975. -
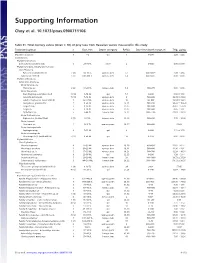
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

The Ecology of Seamounts: Structure, Function, and Human Impacts
University of Plymouth PEARL https://pearl.plymouth.ac.uk Faculty of Science and Engineering School of Biological and Marine Sciences 2010 The ecology of seamounts: structure, function, and human impacts. Clark, MR http://hdl.handle.net/10026.1/1339 10.1146/annurev-marine-120308-081109 Ann Rev Mar Sci All content in PEARL is protected by copyright law. Author manuscripts are made available in accordance with publisher policies. Please cite only the published version using the details provided on the item record or document. In the absence of an open licence (e.g. Creative Commons), permissions for further reuse of content should be sought from the publisher or author. ANRV399-MA02-10 ARI 13 November 2009 17:42 The Ecology of Seamounts: Structure, Function, and Human Impacts Malcolm R. Clark,1 Ashley A. Rowden,1 Thomas Schlacher,2 Alan Williams,3 Mireille Consalvey,1 Karen I. Stocks,4 Alex D. Rogers,5 Timothy D. O’Hara,6 Martin White,7 Timothy M. Shank,8 and Jason M. Hall-Spencer9 1National Institute of Water & Atmospheric Research (NIWA), Wellington 6021, New Zealand; email: [email protected] 2University of the Sunshine Coast, Maroochydore DC, QLD 4558, Australia 3Commonwealth Scientific and Industrial Research Organisation (CSIRO), Wealth from Oceans Flagship, Marine Laboratories, Hobart, Tasmania 7001, Australia 4University of California, San Diego, SDSC, La Jolla, California 92093 5Institute of Zoology, Zoological Society of London, Regents Park, London, NW1 4RY, United Kingdom 6Museum of Victoria, Melbourne 3001, Australia 7Department of Earth and Ocean Sciences, National University of Ireland, Galway, Ireland 8Biology Department, MS33 Redfield Laboratory, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543 9Marine Biology and Ecology Research Centre, Marine Institute, University of Plymouth, Plymouth PL4 8AA, United Kingdom Annu.