Combining Statistical Tools and Ecological Assessments In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inventaire-CIK-09042017.Pdf
Autre(s) Nb de Date Estampages Estampages Autres K. Ext. Ind. Pays, province Lieu d’origine Situation actuelle Type Détails Matériau Langue Éditions/traductions n° lignes (śaka) EFEO BNF est. In situ ; dans la maçonnerie de 1 - - - Vietnam, An Giang Vat Thling l’autel de la pagode du village de Stèle 26 Khmer vie-viie 271 302 (34) - IC VI, p. 28-30 Lê Hoat, Châu Đốc Dalle formant seuil du tombeau du BEFEO XL, p. 480 2 - - - Vietnam, An Giang Phnom Svam ou Nui Sam In situ (en 1923, Cœdès 1923) Dalle 12 Sanskrit xe - 301 (34) - mandarin Nguyên (chronique) Ngoc Thoai ; Ruinée Trouvée sur l'allée In situ (en 2002), enchassé dans 303 (34) ; 471 3 - - - Vietnam, An Giang Linh Son Tu Piédroit principale de Linh Schiste ardoisier 11 Sanskrit vie-viie n. 295 - Cœdès 1936, p. 7-9 l'autel de Linh Son Tu (79) Son Tu 4 - - - Vietnam, An Giang Linh Son Tu Inconnue Stèle Ruinée 12 Khmer xe - 304 (34) - - Vietnam, Đồng Thap Muoi, Prasat Pram BTLSHCM 2893 (Kp 1, 2) ; 305 (69) ; 472 5 - - - Piédroit Schiste ardoisier 22 Sanskrit ve 329 ; n. 15 - Cœdès 1931, p. 1-7 Tháp Loveng BTLS 5964? (79) Vietnam, Đồng Thap Muoi, Prasat Pram 6 - - - Inconnue Stèle 10 Khmer vie-viie - 306 (34) - Cœdès 1936, p. 5 Tháp Loveng Vietnam, Đồng Thap Muoi, Prasat Pram Chapiteau 307 (34) ; 473 7 - - - Inconnue 20 Khmer vie-viie 331 ; n. 16 - Cœdès 1936, p. 3-5 Tháp Loveng (?) (79) Vietnam, Đồng Thap Muoi, Prasat Pram 308 (34) ; 474 8 - - - DCA 6811 Piédroit Schiste ardoisier 10 Khmer vie-viie 259 ; n. -

Cambodia-10-Contents.Pdf
©Lonely Planet Publications Pty Ltd Cambodia Temples of Angkor p129 ^# ^# Siem Reap p93 Northwestern Eastern Cambodia Cambodia p270 p228 #_ Phnom Penh p36 South Coast p172 THIS EDITION WRITTEN AND RESEARCHED BY Nick Ray, Jessica Lee PLAN YOUR TRIP ON THE ROAD Welcome to Cambodia . 4 PHNOM PENH . 36 TEMPLES OF Cambodia Map . 6 Sights . 40 ANGKOR . 129 Cambodia’s Top 10 . 8 Activities . 50 Angkor Wat . 144 Need to Know . 14 Courses . 55 Angkor Thom . 148 Bayon 149 If You Like… . 16 Tours . 55 .. Sleeping . 56 Baphuon 154 Month by Month . 18 . Eating . 62 Royal Enclosure & Itineraries . 20 Drinking & Nightlife . 73 Phimeanakas . 154 Off the Beaten Track . 26 Entertainment . 76 Preah Palilay . 154 Outdoor Adventures . 28 Shopping . 78 Tep Pranam . 155 Preah Pithu 155 Regions at a Glance . 33 Around Phnom Penh . 88 . Koh Dach 88 Terrace of the . Leper King 155 Udong 88 . Terrace of Elephants 155 Tonlé Bati 90 . .. Kleangs & Prasat Phnom Tamao Wildlife Suor Prat 155 Rescue Centre . 90 . Around Angkor Thom . 156 Phnom Chisor 91 . Baksei Chamkrong 156 . CHRISTOPHER GROENHOUT / GETTY IMAGES © IMAGES GETTY / GROENHOUT CHRISTOPHER Kirirom National Park . 91 Phnom Bakheng. 156 SIEM REAP . 93 Chau Say Tevoda . 157 Thommanon 157 Sights . 95 . Spean Thmor 157 Activities . 99 .. Ta Keo 158 Courses . 101 . Ta Nei 158 Tours . 102 . Ta Prohm 158 Sleeping . 103 . Banteay Kdei Eating . 107 & Sra Srang . 159 Drinking & Nightlife . 115 Prasat Kravan . 159 PSAR THMEI P79, Entertainment . 117. Preah Khan 160 PHNOM PENH . Shopping . 118 Preah Neak Poan . 161 Around Siem Reap . 124 Ta Som 162 . TIM HUGHES / GETTY IMAGES © IMAGES GETTY / HUGHES TIM Banteay Srei District . -
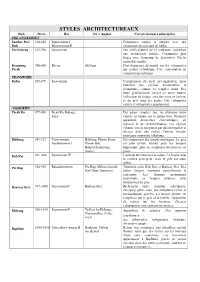
Styles Architectureaux
STYLES ARCHITECTUREAUX Style Dates Roi Où à Angkor Caractéristiques principales PRE-ANGKORIEN Sambor Prei 610-650 Isanavarman I, Colonnettes rondes et simples avec des Kuk Bhavavarman II chapiteaux incorporant un bulbe. Prei Kmeng 635-700 Jayavarman Des chefs-d'œuvre de la sculpture, cependant une architecture limitée. Colonnettes plus larges avec beaucoup de décoration. Déclin général de qualité Kompong 700-800 Divers AkYum Plus d'anneaux décoratifs sur les colonnettes Preah qui restent cylindrique. Une continuation de construction en brique TRANSITOIRE Kulen 825-875 Jayavarman Continuation du style pré-angkorien, mais toutefois une période d'innovation et d'emprunts, comme les temples cham. Des tours généralement carrées et assez hautes. Utilisation de brique, avec des murs en latérite, et du grès pour les portes. Des colonnettes carrées et octogonales apparaissent. ANGKORIEN Preah Ko 877-886 Preah Ko, Bakong, Des plans simples: une ou plusieurs tours Lolei carrées en brique sur la même base. Première apparition d'enceintes concentriques, de gopuras et de «bibliothèques». Les «palais volants» ont été remplacés par des dvarapalas et devatas dans des niches. Premier temple- montagne important à Bakong. Bakheng 889-923 Yasovarman I, Bakheng, Phnom Krom, Développement des temple-montagnes. Le grès Harshavarman I Phnom Bok, est plus utilisé, surtout pour les temples Baksei Chamkrong importants; plus de sculptures décoratives en (trans.) pierre. Koh Ker 921 -944 Jayavarman IV L'échelle diminue vers le centre. La brique reste la matière principale, mais le grès est aussi utilisé. Prè Rup 944-968 Râjendravarman Prè Rup, Mébon oriental, Transition entre Koh Ker et Banteay Srei. Des Bat Chum, Kutisvara halles longues entourent partiellement le sanctuaire. -

Destination: Angkor Archaeological Park the Complete Temple Guide
Destination: Angkor Archaeological Park The Complete Temple Guide 1 The Temples of Angkor Ak Yom The earliest elements of this small brick and sandstone temple date from the pre-Angkorian 8th century. Scholars believe that the inscriptions indicate that the temple is dedicated to the Hindu 'god of the depths'. This is the earliest known example of the architectural design of the 'temple-mountain', which was to become the primary design for many of the Angkorian period temples including Angkor Wat. The temple is in a very poor condition. Angkor Thom Angkor Thom ("Great City") was the last and most enduring capital city of the Khmer empire. It was established in the late 12th century by King Jayavarman VII. The walled and moated royal city covers an area of 9 km², within which are located several monuments from earlier eras as well as those established by Jayavarman and his successors. At the centre of the city is Jayavarman's state temple, the Bayon, with the other major sites clustered around the Victory Square immediately to the north. Angkor Thom was established as the capital of Jayavarman VII's empire, and was the centre of his massive building programme. One inscription found in the city refers to Jayavarman as the groom and the city as his bride. Angkor Thom is accessible through 5 gates, one for each cardinal point, and the victory gate leading to the Royal Palace area. Angkor Wat Angkor Wat ("City of Temples"), the largest religious monument in the world, is a masterpiece of ancient architecture. The temple was built by the Khmer King Suryavarman II in the early 12th century as his state temple and eventual mausoleum. -

Japanese Government Team for Angkor Safeguarding
Japanese Government Team for Safeguarding Angkor ユネスコ文化遺産保存日本信託基金による国際協力活動 International Cooperation Activities by UNESCO / JAPAN Trust Fund for the Preservation of World Cultural Heritage 日本国政府アンコール遺跡救済チーム JSA による保存修復が行われている遺跡 Map of sites benefited from JSA バイヨン寺院の北経蔵 アンコール・トム王宮前広場にある Northern Library プラサート・スープラとそのテラス of the Bayon Temple Prasat Suor Prat and terraces at the Royal Plaza of Angkor Thom 北経蔵 JSA バイヨン展示小屋 Bayon Exhibition Hut of JSA Northern Library プラサート・ スープラ北群 Prasat Suor Prat Northern side 王宮前広場 Royal Plaza 王宮 N1 塔 /N1ETower Royal Palace N2 塔 /N2ETower プラサート・ スープラ南群 N N Prasat Suor Prat Southern side Banteay Srei Phnom Kulen Phnom Bok Preah Khan Neak Pean Ta Som Elephant terrace Ta Nei Thommanon EAST BARAY Phimeanakas バイヨン Ta Keo East Mebon Baphuon BAYON Chau Say Tevoda Banteay Samre WEST BARAY アンコール・トム West Mebon Ta Phrohm ANGKOR THOM Pre Rup Banteay Kdei Phnom Bakheng アンコール・ワット Ak Yom ANGKOR WAT Prasat Kravan 外周壁内北経蔵 Northern Library inside the outermost Enclosure アンコールセンチュリーホテル ANGKOR CENTURY HOTEL N グランドホテル GRAND HOTEL D’s ANGKOR N 0 1 2 3 4 5km シェムリアップ SIEM REAP JSA シェムリアップオフィス/ JSA Siem Reap Office アンコール・ワットの最外周壁内にある北経蔵 アンコールセンチュリーホテルより北へ車で 2 分 2 minutes north from Angkor Century Hotel by a car Northern Library inside the Outermost Enclosure of Angkor Wat バイヨン Bayon 王宮前広場とプラサート・スープラ アンコール・ワット Angkor Wat The Royal Plaza and Prasat Suor Prat −1− 心に沁みる修復を! Restoration that touches your heart! NAKAGAWA Takeshi 中川 武(JSA 団長/早稲田大学教授) (Director General of JSA / Prof. of Waseda Univ.) 1980年代末よりカンボジア和平に主導的な役割を果たした日本 政府は、その後の社会復興のために継続的な国際協力が不可欠と Japanese Government that had played a leading role in the peace negotiation of Cambodia from the late 1980’s, thought it was essential to make a continuous inter- 考え、その象徴的事業として、日仏の協力のもとに、国際協調の national cooperation for their postwar social rehabilitation. -

A STUDY of the NAMES of MONUMENTS in ANGKOR (Cambodia)
A STUDY OF THE NAMES OF MONUMENTS IN ANGKOR (Cambodia) NHIM Sotheavin Sophia Asia Center for Research and Human Development, Sophia University Introduction This article aims at clarifying the concept of Khmer culture by specifically explaining the meanings of the names of the monuments in Angkor, names that have existed within the Khmer cultural community.1 Many works on Angkor history have been researched in different fields, such as the evolution of arts and architecture, through a systematic analysis of monuments and archaeological excavation analysis, and the most crucial are based on Cambodian epigraphy. My work however is meant to shed light on Angkor cultural history by studying the names of the monuments, and I intend to do so by searching for the original names that are found in ancient and middle period inscriptions, as well as those appearing in the oral tradition. This study also seeks to undertake a thorough verification of the condition and shape of the monuments, as well as the mode of affixation of names for them by the local inhabitants. I also wish to focus on certain crucial errors, as well as the insufficiency of earlier studies on the subject. To begin with, the books written in foreign languages often have mistakes in the vocabulary involved in the etymology of Khmer temples. Some researchers are not very familiar with the Khmer language, and besides, they might not have visited the site very often, or possibly also they did not pay too much attention to the oral tradition related to these ruins, a tradition that might be known to the village elders. -

Nature and Provenance of the Sandstone Used for Bayon Style Sculptures Produced During the Reign of Jayavarman VII
Journal of Archaeological Science 40 (2013) 723e734 Contents lists available at SciVerse ScienceDirect Journal of Archaeological Science journal homepage: http://www.elsevier.com/locate/jas Nature and provenance of the sandstone used for Bayon style sculptures produced during the reign of Jayavarman VII Federico Carò a,*, Janet G. Douglas b a Department of Scientific Research, The Metropolitan Museum of Art, 1000 Fifth Avenue, New York, NY 10028, United States b Department of Conservation and Scientific Research, Freer Gallery of Art/Arthur M. Sackler Gallery, Smithsonian Institution, Washington, DC 20560, United States article info abstract Article history: Under Jayavarman VII (1182/83-ca.1218 CE) the Khmer empire reached its apex, leaving a heritage of Received 13 June 2012 major construction works and unique artistic production. The stone materials of several sculptures Accepted 16 June 2012 produced under his reign were characterized and compared to possible geological sources in northern and eastern Cambodia. The data suggest that a specific type of sandstone, rich in volcanic detritus, was Keywords: deliberately selected and quarried from a Triassic sedimentary sequence exposed far from Angkor, the Petrography main political and economic center at that time. Volcanic grains Ó 2012 Elsevier Ltd. All rights reserved. Sandstone Sculptures Bayon Angkor Cambodia 1. Introduction The provenance of this sandstone and the location of the workshop have been the subject of much speculation. Some Under the reign of Jayavarman VII (1182/83-ca.1218 CE) many scholars have placed the source of stone used for both architecture significant construction projects were undertaken, such as major and sculpture of Bayon style in Phnom Kulen (Kulen Mountains), or roads, stone bridges, hospitals and temples, which testify to a deep more generically among the sandstones belonging to the Khorat interaction with the local environment and knowledge of its series (Delvert, 1963; Woodward, 1980; Jessup and Zephir, 1997). -

East of Angkor Description Some 13 Kilometers East of Siem Reap, on the Road to Phnom Penh, Is the Roluos Group of Temples
east of angkor description some 13 kilometers east of siem reap, on the road to phnom penh, is the roluos group of temples. this is the destination for the afternoon’s outing and transport from amansara by jeep can be arranged for about 3.00pm. the group comprises of three main temples, lolei, which is surrounded by an active wat, preah ko and bakong. jayavarman II chose hariharalaya (present day roluos) as one of the first capital cities of the angkor period in the 9th century. it is a great starting point for early angkorian exploration. the setting is somewhat quieter than the more popular and frequented temples within the park and is ideal for contemplative time. returning to amansara by a country road which provides the opportunity to see traditional rural life is a highlight in itself. in addition, on the road home there is one more small temple complex for the true enthusiasts, prei monti with a most unusual stone bathing pool. recent excavations suggest this may have been the site of the hariharalaya royal palace. you will arrive home at approximately 6.00pm. guests arriving into siem reap on a morning flight may wish to get started on their outings a little earlier. recommended departure for east of angkor is just after lunch with the addition of the remote temple ruin of trapeang toteung thngai. although very little of the temple remains the setting at the edge of the great lake is extremely picturesque. this hindu temple, set in a bamboo forest, was possibly founded under the reign of jayavarman III and built during the 9th century. -

Camboya Más Vendida
“ Aquí se puede ascender al reino de los dioses Para conocer la en Angkor Wat y bajar esencia del lugar a los infi ernos en la prisión de Tuol Sleng. Experienciass El presente de Camboya Camboya asombrosas Con fotografías es la embriagadora sugerentes, los lugareses imprescindibles y todaa mezcla de una historia la información de esperanzadora y primera mano. Camboya depresiva a la vez.” Organizar NICK RAY, el viaje AUTOR DE LONELY PLANET perfecto Herramientas para planifi car la visita e itinerarios para preparar el viaje ideal. Más allá de las rutas trilladas Nuestros autores desvelan los secretos locales que harán del viaje algo único. Cobertura especial Mapas de los templos de Angkor Actividades al aire libre Comida y bebida Viaje responsable Nuestro compromiso Los autores de Lonely Planet visitan los lugares sobre Los mejores los que escriben en cada edición y nunca aceptan Escanear consejos regalos a cambio de reseñas favorables: lo explican este código Nº todo tal como lo ven. para más LA GUÍA 1DE información. Los lugares OTROS TÍTULOS DE LA COLECCIÓN emblemáticos CAMBOYA CONSÚLTESE EL INTERIOR O ir a la web de Lonely MÁS VENDIDA en detalle VÉASE INTERIOR Planet en busca de CUBIERTA consejos, información Recomendaciones 10137197 y reservas de viajes. de expertos PVP. 25,00PVP. € Camboya EDICIÓN ESCRITA Y DOCUMENTADA POR Nick Ray, Jessica Lee 00-sumario.indd 1 21/10/16 12:01 PUESTA A PUNTO EXPLORAR Bienvenidos PHNOM PENH ......44 Kompong Khleang ......136 a Camboya ............. 4 Puntos de interés ........ 48 Me Chrey ..............136 Mapa de Camboya .......6 Actividades ............. 58 Las 10 mejores Cursos ................ -

Moss Flora of Angkor, Cambodia
Bull. Natl. Mus. Nat. Sci., Ser. B, 35(3), pp. 141–150, September 22, 2009 Moss Flora of Angkor, Cambodia Masanobu Higuchi Department of Botany, National Museum of Nature and Science, Amakubo 4–1–1, Tsukuba 305–0005, Japan E-mail: [email protected] Abstract The moss flora of Angkor, Cambodia, was investigated in 2005 and 2006 with special attention to Ta Nei site. The bryophytes recognized comprise 11 families, 14 genera and 19 species. Calymperes motleyi, Ectropothecium dealbatum, Fissidens crenulatus, F. flaccidus, F. teysmannianus, Hyophila rosea, Leptobryum pyriforme, Scopelophila cataractae and Splachno- bryum flaccidum are reported as new to the moss flora of Cambodia. For each species recognized here, locality, substrate, specimen number and taxonomic note are provided. Key words : Angkor, Cambodia, mosses, Ta Nei. study is to investigate the moss flora of Angkor Introduction and compile it based on the specimens collected. This study deals with the moss flora of Angkor, Cambodia based on the collections Materials and Methods under a research program, “Research on the dete- rioration and conservation of materials constitut- Field studies were carried out in December ing cultural heritage in Asia,” by the National 2005 and July 2006 and a total of ca. 100 speci- Research Institute for Cultural Properties, Tokyo, mens were collected. The sites investigated are Japan in collaboration with the Authority for the divided as follows. The collections are preserved Protection and Management of Angkor and the in the herbarium of the National Museum of Region of Siem Reap, Cambodia. In 2005 and Nature and Science (TNS). 2006 I made field researches and collected I: Prasat Suor Prat, 30 m alt., 13°27ЈN, 103°53ЈE, bryophytes mainly from Ta Nei site (13°27ЈN, December 15, 2005. -

4-Day Experience Siem Reap by Tuk Tuk and Bicycle
4-day Experience Siem Reap by Tuk Tuk and Bicycle Downloaded on: 5 Oct 2021 Tour code: REPDSBSB Tour type ( Private ) Tour Level: Relaxed / Easy Tour Comfort: Standard Tour Period: 4 Days English,German,French,Spanish Siem Reap highlights tour details Explore the 1200 year-old Roluos Group of temples Begin your tour with a visit to the Roluos Group monuments, before Discover the temples of Angkor Wat by tuk tuk continuing your temple visits by Tuk Tuk. You'll visit Prasat Kravan, Witness spectacular Cambodian sunsets the Srah Srang reservoir, Banteay Kdei, Eastern Mebon & the Pre Rup Take a boat trip down Tonle Sap Lake mountain temple at sunset. Visit a local floating village You'll also visit the South Gate, the ancient capital of Angkor Thom, Enjoy a traditional Apsara show Bayon temple (unique for its 200 'smiling faces'), Baphuon & the Elephants Terrace. Finish your day with a sunset visit to Angkor Wat! The following day you'll set out on a bicycle adventure to the temples of Preah Khan, Ta Nei temple & Ta Prohm. You'll then drive to Banteay Srei temple before stopping at a local village to observe traditional palm sugar production. Finish with a visit to Banteay Samre, an Apsara Dance Show, an excursion to a floating village & a boat trip down Tonle Sap Lake. Contact [email protected] www.diethelmtravel.com Copyright © Diethelm Travel Management Limited. All right reserved. 4-day Experience Siem Reap by Tuk Tuk and Bicycle Contact [email protected] www.diethelmtravel.com Copyright © Diethelm Travel Management Limited. -

Page 1 of 3 #678, Group 1, Phum Tavien, Siem Reap City, CAMBODIA H/P: +855 12 971 645 E-Mail: [email protected] Info@Cambodiatrav
Page 1 of 3 #678, Group 1, Phum Tavien, Siem Reap City, CAMBODIA H/P: +855 12 971 645 E-mail: [email protected] [email protected] Website: www.cambodiatraveltrails.com -------------------------------------------------------------------------------------------------------------------------------------------------- CYCLING OPTION 1: ANGKOR WAT BAYON PREAH KHAN TA NEI TAPROHM Tour Code: C1 Distance: 40 km Duration: 8 hours Group Size: 1 - 10 pax Daily: 8:00AM - 4:00PM Tour Price: USD40 per person OVERVIEW Deep in the forests of Cambodia’s Siem Reap province, the elegant spires of an ancient stone city soar skyward above the sprawling complex of Angkor Archaeological Park. Angkor served as the capital of the Khmer Empire (9th - 15th centuries), which ruled the region at the time. While there are over 100 stone temples; there are 6 most impressive ones that are the definite must-sees. Those include: Angkor Wat (the one appears on Cambodia national flag) Bayon Preah Khan Tanei Taprom Prasat Chrung Meanwhile, going for a bike ride is a great adventure as well as a great way to avoid the crowds. Our skillful team will guide you through the rainforest trails to explore the stunning un-touristic jungle sites. The tour is for rider of every level since your tour guide will facilitate depending on your abilities of riding. TOUR HIGHLIGHT - Ride through the hidden trails, instead of busy touristic/traffic route - Visit Angkor Wat, one of the seven wonders of the ancient world - Visit South Gate of Angkor Thom City, the most appealing defensive