Diptera: Acalyptrata)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insects of Ojibway Prairie, a Southern Ontario Tallgras Prairie
199 Chapter 9 Insects of Ojibway Prairie, a Southern Ontario Tallgrass Prairie Steve M. Paiero and Stephen A. Marshall Department of Environmental Biology, University of Guelph Guelph, Ontario, Canada Paul D. Pratt Windsor Department of Parks Windsor, Ontario, Canada Matthias Buck Department of Environmental Biology, University of Guelph Guelph, Ontario, Canada Abstract. This chapter describes the insect fauna of Ojibway Prairie, a tallgrass prairie complex in southern Ontario, highlighting the tallgrass-dependent and tallgrass-associated species among the over 2,000 insect species found there so far. The presence of tallgrass-dependent and tallgrass-associated species reflects Ojibway Prairie’s status as a fragment of a formerly more continuous grassland and thus supports the prairie peninsula hypothesis. The chapter includes a discussion of insect species associated with other southern Ontario tallgrass prairie sites and compares these species with those found in Ojibway Prairie. Also discussed are rare species found at Ojibway Prairie but not associated specifically with tallgrass habitats. Forty-four insect species new to Canada or new to Ontario (1 Orthoptera, 3 Hemiptera, 10 Coleoptera, 16 Diptera, and 14 Hymenoptera) are recorded from Ojibway Prairie. Résumé. Ce chapitre décrit l’entomofaune de la prairie Ojibway, un complexe de prairies à herbes hautes du sud de l’Ontario, en portant une attention particulière aux espèces dépendantes des herbes hautes ou associées à ces dernières et qui sont au nombre des quelque 2 000 espèces d’insectes recensées jusqu’ici à cet endroit. La présence d’insectes dépendants des herbes hautes ou associés à ces dernières est un reflet de l’état actuel de la prairie Ojibway, qui n’est plus qu’un fragment d’une prairie autrefois plus continue, et vient appuyer l’hypothèse de la « péninsule de prairie ». -

Family Descriptions
FAMILY DESCRIPTIONS CAT = Although they do not contain keys, the identification references include recent cata- logues as valuable source on genera, species, distribution and references. CMPD = Contributions to a Manual of Palaearctic Diptera. Lindner = Chapter in Lindner, E., Die Fliegen der Paläarktischen Region. ( ) Family names between brackets refer to names as found in the literature, not recognised here as a separate family but, as indicated, considered part of another family. et al. References with more than two authors are given as First author et al. As far as not yet outdated, the number of genera and species in Europe is largely based on the Catalogue of Palaearctic Diptera, the CMPD and Fauna Europaea, the latter available online at: www.faunaeur.org (consulted was version 1.2, updated 7 March 2005). As to size, the following categories are distinguished: minute: smaller than 2 mm; small: 2- 5 mm; medium sized: 5-10 mm; large: 10-20 mm; very large: over 20 mm. Acartophthalmidae (key couplet 113; fig. 243) Systematics: Acalyptrate Brachycera; superfamily Opomyzoidea; in Europe 1 genus, Acartophthalmus, with 3 species. Characters: Minute to small (1-2.5 mm), brownish grey flies. Arista pubescent, ocelli present; Oc-bristles present; P-bris- tles strong, far apart, diverging; 3 pairs of F-bristles, curving obliquely out-backward, increasing in size, the upper pair the largest; scattered interfrontal setulae present; vibrissae absent but with a series of strong bristles near the vibrissal angle. Wing unmarked or tinged along costa; costa with a humeral break only; vein Sc complete; crossvein BM-Cu present; cell cup closed. -

1 a Checklist of Arthropods Associated with Rat Carrion in a Montane Locality
1 A checklist of arthropods associated with rat carrion in a montane locality of northern Venezuela. Yelitza Velásquez Laboratorio de Biología de Organismos, Centro de Ecología, Instituto Venezolano de Investigaciones Científicas. Apartado Postal 21827, Caracas 1020-A, Venezuela Tel.: +58-212-504.1052; fax: +58-212-504.1088; e-mail: [email protected] Abstract This is the first report of arthropods associated with carrion in Venezuela, using laboratory bred rats (Rattus norvegicus). Rat carcasses were exposed to colonization by arthropods in neighboring montane savanna and cloud forest habitats in the state of Miranda. The taxonomic composition of the arthropods varied between both ecosystems. Scarabaeidae, Silphidae, Micropezidae, Phoridae, Vespidae and one species of ant, were collected only in the cloud forest. Dermestes maculatus, Chrysomya albiceps, Termitidae and most species of ants, were found only in the savanna. Fourteen species were considered to be of primary forensic importance: Dermestes maculatus, Oxelytrum discicolle, Calliphora sp., Cochliomyia macellaria, Compsomyiops sp., Chrysomya albiceps, Phaenicia cuprina, P. sericata, P. eximia, Fannia sp., Puliciphora sp., Megaselia scalaris, Ravina sp. and Sarcophaga sp. Key words: Coleoptera, Diptera, Forensic entomology, Venezuela. Introduction There is relatively little information available regarding insects associated with animal carrion and human corpses in South America [1]. In Brazil, Moura et al. [2] made a preliminary analysis of the insects of medico-legal importance in Curitiba, in the state of 2 Paraná; Carvalho et al. [3] identified arthropods associated with pig carrion and human corpses in Campinas, in the state of São Paulo. Recently, forensic entomology was applied to estimate the postmortem interval (PMI) in homicide investigations by the Rio de Janeiro Police Department, Brasil [4]. -

Proceedings of the United States National Museum
REVISION OF THE TWO-WINGED FLIES OF THE FAMILY CLUSIIDAE. By A. L. Melander and Naomi George Argo, Of the State College of Washington, Pullman. The family Clusiidae, sometimes called the Heteroneuridae or the Clusiodidae, is generally regarded as one of the rarer groups of the Diptera. Seldom are its members met with in more than solitary individuals. In our experience in collecting a hundred thousand specimens of Diptera but a few dozen representatives of Clusiidae have been encountered. Previously there have been described from the entire world 13 valid genera and 55 species. Aldrich's Catalogue in 1905 listed but 2 genera and 12 species as known from North America. The sub- sequent publications of Johnson and Malloch have added 11 recog- nized new species to the American list. Fifteen species have been described from Europe and the same number from South America, while four species have been recorded from the islands south of Asia. The material secured for the present study, amounting to some 400 specimens, has produced 52 species, of which 25 are new. With the extension in distribution of species originally described from Europe or South America there are now known to occur in North America, including Central America, a total of 58 species belonging to 7 genera. Thus in its present status the family includes 80 recognized species distributed among 13 genera. The Clusiidae are restricted in their distribution to Europe, North and South America, and the East Indies. No species have been described from Africa, Australia, or Asia, but there is mention by Lefroy of the occurrence of an undetermined species in India. -
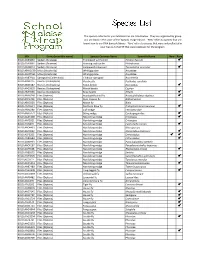
Species List
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 59 that were deployed for the program. BIN Group (scientific name) Species Common Name Scientific Name New Rare BOLD:AAB3890 Spiders (Araneae) Thinlegged wolf spider Pardosa fuscula BOLD:AAA9991 Spiders (Araneae) Running crab spider Philodromus BOLD:AAB8024 Spiders (Araneae) Longjawed orbweaver Tetragnatha versicolor BOLD:ABW2639 Mites (Arachnida) Whirligig mite Anystidae BOLD:AAM7961 Mites (Arachnida) Whirligig mite Anystidae BOLD:AAB7914 Springtails (Collembola) Globular springtail Bourletiella BOLD:ABX3225 Beetles (Coleoptera) Flea beetle Psylliodes cucullata BOLD:ABA0187 Beetles (Coleoptera) Snout beetle Dorytomus BOLD:AAG3633 Beetles (Coleoptera) Marsh beetle Cyphon BOLD:ABA9956 Beetles (Coleoptera) Rove beetle Atheta BOLD:AAN6240 Flies (Diptera) Acartophthalmid fly Acartophthalmus nigrinus BOLD:ACP6334 Flies (Diptera) Root maggot fly Anthomyiinae BOLD:ABV0350 Flies (Diptera) March fly Bibio BOLD:AAC9614 Flies (Diptera) Northern blow fly Protophormia terraenovae BOLD:ABX0280 Flies (Diptera) Gall midge Cecidomyiidae BOLD:AAN5172 Flies (Diptera) Biting midge Ceratopogonidae BOLD:AAA5300 Flies (Diptera) Non-biting midge Cricotopus BOLD:AAP5921 Flies (Diptera) Non-biting midge Cricotopus BOLD:AAI9470 Flies (Diptera) Non-biting midge Cyphomella -

Diptera – Brachycera
Biodiversity Data Journal 3: e4187 doi: 10.3897/BDJ.3.e4187 Data Paper Fauna Europaea: Diptera – Brachycera Thomas Pape‡§, Paul Beuk , Adrian Charles Pont|, Anatole I. Shatalkin¶, Andrey L. Ozerov¶, Andrzej J. Woźnica#, Bernhard Merz¤, Cezary Bystrowski«», Chris Raper , Christer Bergström˄, Christian Kehlmaier˅, David K. Clements¦, David Greathead†,ˀ, Elena Petrovna Kamenevaˁ, Emilia Nartshuk₵, Frederik T. Petersenℓ, Gisela Weber ₰, Gerhard Bächli₱, Fritz Geller-Grimm₳, Guy Van de Weyer₴, Hans-Peter Tschorsnig₣, Herman de Jong₮, Jan-Willem van Zuijlen₦, Jaromír Vaňhara₭, Jindřich Roháček₲, Joachim Ziegler‽, József Majer ₩, Karel Hůrka†,₸, Kevin Holston ‡‡, Knut Rognes§§, Lita Greve-Jensen||, Lorenzo Munari¶¶, Marc de Meyer##, Marc Pollet ¤¤, Martin C. D. Speight««, Martin John Ebejer»», Michel Martinez˄˄, Miguel Carles-Tolrá˅˅, Mihály Földvári¦¦, Milan Chvála ₸, Miroslav Bartákˀˀ, Neal L. Evenhuisˁˁ, Peter J. Chandler₵₵, Pierfilippo Cerrettiℓℓ, Rudolf Meier ₰₰, Rudolf Rozkosny₭, Sabine Prescher₰, Stephen D. Gaimari₱₱, Tadeusz Zatwarnicki₳₳, Theo Zeegers₴₴, Torsten Dikow₣₣, Valery A. Korneyevˁ, Vera Andreevna Richter†,₵, Verner Michelsen‡, Vitali N. Tanasijtshuk₵, Wayne N. Mathis₣₣, Zdravko Hubenov₮₮, Yde de Jong ₦₦,₭₭ ‡ Natural History Museum of Denmark, Copenhagen, Denmark § Natural History Museum Maastricht / Diptera.info, Maastricht, Netherlands | Oxford University Museum of Natural History, Oxford, United Kingdom ¶ Zoological Museum, Moscow State University, Moscow, Russia # Wrocław University of Environmental and Life Sciences, Wrocław, -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Holland Landing Prairie Provincial Park
HOLLAND LANDING PRAIRIE PROVINCIAL PARK One Malaise trap was deployed at Holland Landing Prairie Provincial Park in 2014 (44.11894, -79.48795, 271m ASL; Figure 1). This trap collected arthropods for twenty weeks from May 1 – September 22, 2014. All 10 Malaise trap samples were processed using the bulk sample analysis protocol. A total of 2345 BINs were obtained. Over half the BINs captured were flies (Diptera), followed by bees, ants and wasps (Hymenoptera), moths and butterflies (Lepidoptera), and true bugs (Hemiptera; Figure 2). In total, 544 arthropod species were named, representing 26.1% of the BINs from the site (Appendix 1). All the BINs were assigned at least to family, and 60.5% were assigned to a genus Figure 1. Malaise trap deployed at Holland Landing (Appendix 2). Specimens collected from Holland Prairie Provincial Park in 2014. Landing Prairie represent 165 different families and 623 genera. Figure 2. Taxonomy breakdown of BINs captured in the Malaise trap at Holland Landing Prairie. APPENDIX 1. TAXONOMY REPORT Class Order Family Genus Species Arachnida Araneae Theridiidae Theridion Theridion murarium Diplopoda Julida Julidae Insecta Psocodea Amphipsocidae Polypsocus Polypsocus corruptus Caeciliusidae Valenzuela Valenzuela burmeisteri Valenzuela flavidus Valenzuela nadleri Ectopsocidae Ectopsocus Ectopsocus meridionalis Lachesillidae Lachesilla Lepidopsocidae Echmepteryx Echmepteryx hageni Peripsocidae Peripsocus Peripsocus madidus Peripsocus subfasciatus Psocidae Cerastipsocus Trichadenotecnum Trichadenotecnum alexanderae -

Beiträge Zur Bayerischen Entomofaunistik 13: 67–207
Beiträge zur bayerischen Entomofaunistik 13:67–207, Bamberg (2014), ISSN 1430-015X Grundlegende Untersuchungen zur vielfältigen Insektenfauna im Tiergarten Nürnberg unter besonderer Betonung der Hymenoptera Auswertung von Malaisefallenfängen in den Jahren 1989 und 1990 von Klaus von der Dunk & Manfred Kraus Inhaltsverzeichnis 1. Einleitung 68 2. Untersuchungsgebiet 68 3. Methodik 69 3.1. Planung 69 3.2. Malaisefallen (MF) im Tiergarten 1989, mit Gelbschalen (GS) und Handfänge 69 3.3. Beschreibung der Fallenstandorte 70 3.4. Malaisefallen, Gelbschalen und Handfänge 1990 71 4. Darstellung der Untersuchungsergebnisse 71 4.1. Die Tabellen 71 4.2. Umfang der Untersuchungen 73 4.3. Grenzen der Interpretation von Fallenfängen 73 5. Untersuchungsergebnisse 74 5.1. Hymenoptera 74 5.1.1. Hymenoptera – Symphyta (Blattwespen) 74 5.1.1.1. Tabelle Symphyta 74 5.1.1.2. Tabellen Leerungstermine der Malaisefallen und Gelbschalen und Blattwespenanzahl 78 5.1.1.3. Symphyta 79 5.1.2. Hymenoptera – Terebrantia 87 5.1.2.1. Tabelle Terebrantia 87 5.1.2.2. Tabelle Ichneumonidae (det. R. Bauer) mit Ergänzungen 91 5.1.2.3. Terebrantia: Evanoidea bis Chalcididae – Ichneumonidae – Braconidae 100 5.1.2.4. Bauer, R.: Ichneumoniden aus den Fängen in Malaisefallen von Dr. M. Kraus im Tiergarten Nürnberg in den Jahren 1989 und 1990 111 5.1.3. Hymenoptera – Apocrita – Aculeata 117 5.1.3.1. Tabellen: Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 117 5.1.3.2. Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 122 5.1.4. Coleoptera 131 5.1.4.1. Tabelle Coleoptera 131 5.1.4.2. -

December 2016 Volume 55, Number 4 TRI- OLOGY a Publication from the Division of Plant Industry, Bureau of Entomology, Nematology, and Plant Pathology Dr
FDACS-P-00124 October - December 2016 Volume 55, Number 4 TRI- OLOGY A PUBLICATION FROM THE DIVISION OF PLANT INDUSTRY, BUREAU OF ENTOMOLOGY, NEMATOLOGY, AND PLANT PATHOLOGY Dr. Trevor R. Smith, Division Director BOTANY ENTOMOLOGY NEMATOLOGY PLANT PATHOLOGY Providing information about plants: Identifying arthropods, taxonomic Providing certification programs and Offering plant disease diagnoses and native, exotic, protected and weedy research and curating collections diagnoses of plant problems management recommendations Erythemis simplicicollis, Eastern Pondhawk Photo Credit: Jeffrey Weston Lotz, DPI Florida Department of Agriculture and Consumer Services • Adam H. Putnam, Commissioner 1 Erythemis simplicicollis, Eastern Pondhawk Photo Credit: Jeffrey Weston Lotz, DPI ABOUT TRI-OLOGY TABLE OF ContentS The Florida Department of Agriculture and Consumer Services HIGHLIghtS 03 Division of Plant Industry’s Bureau of Entomology, Nematology and Plant Pathology (ENPP), (including the Botany Section), produces Noteworthy examples from the diagnostic groups through- out the ENPP Bureau. TRI-OLOGY four times a year, covering three months of activity in each issue. The report includes detection activities from nursery plant BOTANY 04 inspections, routine and emergency program surveys, and requests Quarterly activity reports from Botany and selected plant for identification of plants and pests from the public. Samples are identification samples. also occasionally sent from other states or countries for identification or diagnosis. ENTOMOLOGY 06 Quarterly activity reports from Entomology and samples HOW to CITE TRI-ology reported as new introductions or interceptions. Section Editor. Year. Section Name. P.J. Anderson and G.S Hodges (Editors). TRI-OLOGY Volume (number): page. [Date you accessed site] NEMATOLOGY 15 For example: S.E. Halbert. -

Surveillance of Priority Terrestrial Invertebrates in Scotland
Scottish Natural Heritage Commissioned Report No. 609 Surveillance of priority terrestrial invertebrates in Scotland COMMISSIONED REPORT Commissioned Report No. 609 Surveillance of priority terrestrial invertebrates in Scotland For further information on this report please contact: Athayde Tonhasca Scottish Natural Heritage Battleby Redgorton PERTH PH1 3EW Telephone: 01738 458671 E-mail: [email protected] This report should be quoted as: Littlewood, N.A. & Stockan, J.A. 2013. Surveillance of priority terrestrial invertebrates in Scotland. Scottish Natural Heritage Commissioned Report No. 609. This report, or any part of it, should not be reproduced without the permission of Scottish Natural Heritage. This permission will not be withheld unreasonably. The views expressed by the author(s) of this report should not be taken as the views and policies of Scottish Natural Heritage. © Scottish Natural Heritage 2013. COMMISSIONED REPORT Summary Surveillance of priority terrestrial invertebrates in Scotland Commissioned Report No. 609 Contractor: N.A. Littlewood & J.A. Stockan Year of publication: 2013 Background Scottish Natural Heritage has been asked by Scottish ministers to implement a strategy for the surveillance of priority habitats and species in Scotland. This report covers the development of such strategies for 55 species of non-marine invertebrates and draws extensively on expert comment from consultees with specialised knowledge of individual species covered. For each species, a report was written to present background information about the status of the species in Scotland together with issues relevant to surveillance, such as ecology, habitat and threats. This is followed by a Surveillance Methodology, outlining measures that can be taken to monitor the species in such a way as to determine trends in population size, range or status. -

F. Christian Thompson Neal L. Evenhuis and Curtis W. Sabrosky Bibliography of the Family-Group Names of Diptera
F. Christian Thompson Neal L. Evenhuis and Curtis W. Sabrosky Bibliography of the Family-Group Names of Diptera Bibliography Thompson, F. C, Evenhuis, N. L. & Sabrosky, C. W. The following bibliography gives full references to 2,982 works cited in the catalog as well as additional ones cited within the bibliography. A concerted effort was made to examine as many of the cited references as possible in order to ensure accurate citation of authorship, date, title, and pagination. References are listed alphabetically by author and chronologically for multiple articles with the same authorship. In cases where more than one article was published by an author(s) in a particular year, a suffix letter follows the year (letters are listed alphabetically according to publication chronology). Authors' names: Names of authors are cited in the bibliography the same as they are in the text for proper association of literature citations with entries in the catalog. Because of the differing treatments of names, especially those containing articles such as "de," "del," "van," "Le," etc., these names are cross-indexed in the bibliography under the various ways in which they may be treated elsewhere. For Russian and other names in Cyrillic and other non-Latin character sets, we follow the spelling used by the authors themselves. Dates of publication: Dating of these works was obtained through various methods in order to obtain as accurate a date of publication as possible for purposes of priority in nomenclature. Dates found in the original works or by outside evidence are placed in brackets after the literature citation.