Quantitative Analysis of Phenibut in Rat Brain Tissue Extracts by Liquid Chromatography–Tandem Mass Spectrometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
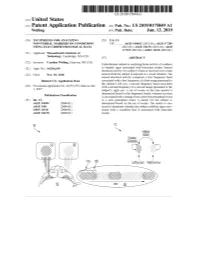
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

EFFECTS of CHRONIC and ACUTE Y-HYDROXYBUTYRATE ADMINISTRATION on MASS and the RELEASE of GROWTH HORMONE and CORTICOSTERONE
EFFECTS OF CHRONIC AND ACUTE y-HYDROXYBUTYRATE ADMINISTRATION ON MASS AND THE RELEASE OF GROWTH HORMONE AND CORTICOSTERONE A Thesis Presented to the Faculty of the College of Science and Technology Morehead State University In Partial Fulfillment of the Requirements for the Degree Master of Science in Biology by Eric C. Goshorn October, 1998 /-t?p-1\'/ I"\s 4 ,he-a ·1 ~ ~lC\ .9~ G ls> 1 lo .JL. Accepted by the faculty of the College of Science and Technology, Morehead State University, in partial fulfillment of the requirements for the Master of Science degree. Master's Committee: /2..-lo -</'?, Date ii EFFECTS OF CHRONIC AND ACUTE y-HYDROXYBUTYRATE ADMINISTRATION ON MASS AND THE RELEASE OF GROWTH HORMONE AND CORTICOSTERONE Eric Christopher Goshorn, M.S. Morehead State University, 1998 Director of Thesis· Gamma-hydroxybutyrate (GHB) is a naturally occurring compound found both in neural (Roth and Suhr 1970) and extraneural tissue (Nelson et al. 1981 ). While the endogenous function of peripheral GHB has yet to be elucidated, experimentation has found that this compound induces anesthesia, and the release of growth hormone and prolactin from the anterior pituitary (Oyama and Takiguchi 1970; Takahara et al. 1977). This study was undertaken to determine the effect of chronic GHB injections on the weight gain of rats during early postnatal development. Acute injections were also administered to rats not previously treated with exogenous GHB to iii ascertain the mechanism of growth hormone release and to determine GHB's effect on corticosterone release. At the age of 3 days, both male and female rats from the chronic group were injected intraperitoneally with 100 mg/kg, 500 mg/kg, or 0 mg/kg of GHB. -
![Muscimol-Scopolamine Interactions in the Rat Brain: a Study with 2-Deoxy-D-[ 1-14C]Glucose1](https://docslib.b-cdn.net/cover/2348/muscimol-scopolamine-interactions-in-the-rat-brain-a-study-with-2-deoxy-d-1-14c-glucose1-1182348.webp)
Muscimol-Scopolamine Interactions in the Rat Brain: a Study with 2-Deoxy-D-[ 1-14C]Glucose1
0270.6474/84/0406-1405$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 6, pp. 1405-1413 Printed in U.S.A. June 1984 MUSCIMOL-SCOPOLAMINE INTERACTIONS IN THE RAT BRAIN: A STUDY WITH 2-DEOXY-D-[ 1-14C]GLUCOSE1 PAUL1 HELEN AND EDYTHE D. LONDON*x2 Laboratory of Neurosciences, National Institute on Aging, Gerontology Research Center, Baltimore City Hospitals, Baltimore, Maryland 21224 and *National Institute on Drug Abuse, Addiction Research Center, c/o Baltimore City Hospitals, Baltimore, Maryland 21224 Received August 22, 1983; Revised November 29, 1983; Accepted December 6, 1983 Abstract The 2-deoxy-D[1-‘4C]glucose method of Sokoloff was used to measure local cerebral glucose utilization (LCGU) in rats after injections of the GABA receptor agonist, muscimol (1.6 mg/kg and 4.0 mg/kg, i.v.); the muscarinic receptor antagonist, scopolamine (0.4 mg/kg and 2.0 mg/kg, i.v.); or combinations of both drugs. The aim was to identify brain regions where functional effects of GABAergic-cholinergic interactions could be seen. As noted previously, muscimol reduced LCGU in many brain regions. In contrast, scopolamine alone had no effect on LCGU in most brain regions; however, decreases were seen in the medial geniculate body, medial thalamic nucleus, and auditory and frontal cortical areas. Scopolamine increased LCGU in the cerebellar vermis and mesencephalic reticular formation. Although muscimol alone did not significantly affect LCGU in the external plexiform layer of the olfactory bulb or the anterior, periventricular, and parafascicular thalamic nuclei, rats treated with 0.4 mg/kg of scopolamine before 4.0 mg/kg of muscimol had LCGU decrements in those brain regions. -

At the Gabaa Receptor
THE EFFECTS OF CHRONIC ETHANOL INTAKE ON THE ALLOSTERIC INTERACTION BE T WEEN GABA AND BENZODIAZEPINE AT THE GABAA RECEPTOR THESIS Presented to the Graduate Council of the University of North Texas in Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE By Jianping Chen, B.S., M.S. Denton, Texas May, 1992 Chen, Jianping, The Effects of Chronic Ethanol Intake on the Allsteric Interaction Between GABA and BenzodiazeDine at the GABAA Receptor. Master of Science (Biomedical Sciences/Pharmacology), May, 1992, 133 pp., 4 tables, 3.0 figures, references, 103 titles. This study examined the effects of chronic ethanol intake on the density, affinity, and allosteric modulation of rat brain GABAA receptor subtypes. In the presence of GABA, the apparent affinity for the benzodiazepine agonist flunitrazepam was increased and for the inverse agonist R015-4513 was decreased. No alteration in the capacity of GABA to modulate flunitrazepam and R015-4513 binding was observed in membranes prepared from cortex, hippocampus or cerebellum following chronic ethanol intake or withdrawal. The results also demonstrate two different binding sites for [3H]RO 15-4513 in rat cerebellum that differ in their affinities for diazepam. Chronic ethanol treatment and withdrawal did not significantly change the apparent affinity or density of these two receptor subtypes. ACKNOWLEDGEMENT I would like to express my sincere thanks to my major professor, Dr. Michael W. Martin. .I deeply appreciate his guidance and direction which initiated this study, and his kindness in sharing his laboratory facilities with me. His suggestions, patience, encouragement and support in the laboratory have contributed significantly to my understanding of the receptor mechanism of drug action. -

World of Cognitive Enhancers
ORIGINAL RESEARCH published: 11 September 2020 doi: 10.3389/fpsyt.2020.546796 The Psychonauts’ World of Cognitive Enhancers Flavia Napoletano 1,2, Fabrizio Schifano 2*, John Martin Corkery 2, Amira Guirguis 2,3, Davide Arillotta 2,4, Caroline Zangani 2,5 and Alessandro Vento 6,7,8 1 Department of Mental Health, Homerton University Hospital, East London Foundation Trust, London, United Kingdom, 2 Psychopharmacology, Drug Misuse, and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom, 3 Swansea University Medical School, Institute of Life Sciences 2, Swansea University, Swansea, United Kingdom, 4 Psychiatry Unit, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy, 5 Department of Health Sciences, University of Milan, Milan, Italy, 6 Department of Mental Health, Addictions’ Observatory (ODDPSS), Rome, Italy, 7 Department of Mental Health, Guglielmo Marconi” University, Rome, Italy, 8 Department of Mental Health, ASL Roma 2, Rome, Italy Background: There is growing availability of novel psychoactive substances (NPS), including cognitive enhancers (CEs) which can be used in the treatment of certain mental health disorders. While treating cognitive deficit symptoms in neuropsychiatric or neurodegenerative disorders using CEs might have significant benefits for patients, the increasing recreational use of these substances by healthy individuals raises many clinical, medico-legal, and ethical issues. Moreover, it has become very challenging for clinicians to Edited by: keep up-to-date with CEs currently available as comprehensive official lists do not exist. Simona Pichini, Methods: Using a web crawler (NPSfinder®), the present study aimed at assessing National Institute of Health (ISS), Italy Reviewed by: psychonaut fora/platforms to better understand the online situation regarding CEs. -

Pharmacokinetics and Pharmacodynamics Of
Liechti et al. Pharmacokinetics and pharmacodynamics of -hydroxybutyrate in healthy subjects Running title: Effects of -hydroxybutyrate in healthy subjects Matthias E. Liechti,1, * Boris B. Quednow,2 Evangelia Liakoni,1 Dario Dornbierer,2 Robin von Rotz,2 M. Salomé Gachet,3 Jürg Gertsch,3 Erich Seifritz,2 Oliver G. Bosch2 1Division of Clinical Pharmacology and Toxicology, Department of Biomedicine and Department of Clinical Research, University Hospital Basel, Βasel, CH-4031, Switzerland 2Department of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zurich, Lenggstrasse 31, Zurich, CH-8032, Switzerland 3Institute of Biochemistry and Molecular Medicine, University of Bern, Bühlstrasse 28, Bern, CH-3012, Switzerland *Correspondence: Dr. Matthias E. Liechti MD, Division of Clinical Pharmacology and Toxicology, University Hospital Basel, Hebelstrasse 2, Basel, CH-4031, Switzerland; Tel: +41 61 328 68 68; Fax: +41 61 265 45 60; E-mail: [email protected] Word count: Abstract: 250 words References: 28 Tables: 2 Figures: 3 This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/bcp.12863 This article is protected by copyright. All rights reserved. Liechti et al. Abstract Aims: -Hydroxybutyrate (GHB) is used as a treatment for narcolepsy and alcohol withdrawal and as recreational substance. Nevertheless, there are limited data on the pharmacokinetics and pharmacokinetic-pharmacodynamic relationship of GHB in humans. We characterized the pharmacokinetic profile and exposure-psychotropic effect relationship of GHB in humans. -

Use of Baclofen in Treatment of Various Medical Conditions
wjpmr, 2017,3(6), 148-151 SJIF Impact Factor: 4.103 WORLD JOURNAL OF PHARMACEUTICAL Research Article Fatima et al. World Journal of Pharmaceutical and Medical ResearchISSN 2455 -3301 AND MEDICAL RESEARCH www.wjpmr.com WJPMR USE OF BACLOFEN IN TREATMENT OF VARIOUS MEDICAL CONDITIONS Dr. Mehraj Fatima*1 and Dr. Ramesh Ganpisetti2 1,2Department of Pharmacy Practice, Malla Reddy Hospital, Hyderabad, Telangana, India-500055. 1,2Faculty of Pharmacy, Malla Reddy of Institute of Pharmaceutical Sciences, Dhullapally, Secunderabad-500100. *Corresponding Author: Dr. Mehraj Fatima Department of Pharmacy Practice, Malla Reddy Hospital, Hyderabad, Telangana, India-500055. Article Received on 29/04/2017 Article Revised on 20/05/2017 Article Accepted on 09/06/2017 ABSTRACT Baclofen, sold under the brand name Lioresal among others, is a central nervous system depressant used as a skeletal muscle relaxant. It is primarily used to treat spasticity. It is also used in topical creams to help with pain. It is a GABA receptor agonist, specifically of the GABAB receptors. Its beneficial effects in spasticity result from actions at spinal and supraspinal sites. It is a derivative of γ-aminobutyric acid (GABA). The drug is rapidly absorbed after oral administration and is widely distributed throughout the body. Biotransformation is low and the drug is predominantly excreted unchanged by the kidneys. The half life of baclofen is roughly 2–4 hours and it therefore needs to be administered frequently throughout the day to control spasticity appropriately. Baclofen comes in tablets of 10 and 20 milligrams (mg), and you'll usually take an equally divided dose three times a day. -
![[3H]-MUSCIMOL, a POTENT Y-AMINOBUTYRIC ACID RECEPTOR AGONIST, to MEMBRANES of the BOVINE RETINA NEVILLE N](https://docslib.b-cdn.net/cover/5654/3h-muscimol-a-potent-y-aminobutyric-acid-receptor-agonist-to-membranes-of-the-bovine-retina-neville-n-3105654.webp)
[3H]-MUSCIMOL, a POTENT Y-AMINOBUTYRIC ACID RECEPTOR AGONIST, to MEMBRANES of the BOVINE RETINA NEVILLE N
Br. J. Pharmac. (1980), 71, 259-264 BINDING OF [3H]-MUSCIMOL, A POTENT y-AMINOBUTYRIC ACID RECEPTOR AGONIST, TO MEMBRANES OF THE BOVINE RETINA NEVILLE N. OSBORNE Nuffield Laboratory of Ophthalmology, The University of Oxford, Walton Street, Oxford OX2 6AW 1 The binding of [3H]-muscimol, a potent y-aminobutyric acid (GABA) receptor agonist, to crude membrane preparations of bovine retina was studied, using a filtration method to isolate membrane- bound ligand. 2 Specific binding was found to be saturable and occurred at two binding sites with affinity con- stants of 4.3 nm and 38.2 nM. 3 Binding was sodium-independent, enhanced by both freezing and Triton X-100 treatment but abolished with sodium laurylsulphate. 4 The binding sites demonstrated a high degree of pharmacological specificity, GABA being a potent displacer of [3H]-muscimol. 5 A higher degree of [3H]-muscimol binding was associated with subcellular fractions enriched with photoreceptor synaptosomes rather than with fractions enriched with conventional synaptosomes. Introduction There is substantial evidence that y-aminobutyric acid lysed, in order to determine the subcellular localiz- (GABA) is a synaptic neuro-transmitter substance in ation of retinal GABA receptors. the vertebrate retina (see Graham 1974 for review). It has been demonstrated that GABA is synthesized and stored in certain neurones in the retina and that the Methods intracellular level can be altered by light (Lam, 1972; very important criterion of whether a sub- Bovine eyes were collected from the abattoir (British 1978). A the stance is a transmitter is to show the existence of Beef Co., Witney, Oxfordshire) within 10min of physiologically relevant receptor sites. -

Methoxy-6-Methylflavone and Kavain at Recombinant GABAA Receptors
Pharmacology profile of 2′-methoxy-6-methylflavone and kavain at recombinant GABAA receptors Han Chow Chua BPharm (Hons) A thesis submitted in fulfilment of the requirements for the award of Doctor of Philosophy Faculty of Pharmacy The University of Sydney 2016 Acknowledgements Firstly, I would like to express my sincere gratitude to my supervisor Professor Mary Collins for her continuous support, guidance, patience and motivation throughout my PhD. Besides my supervisor, I would like to thank Professor Jane Hanrahan, Dr Nathan Absalom and Dr Petra van Nieuwenhuijzen for their insightful comments and encouragement, which were precious for the writing of this thesis. My sincere appreciation also goes to Associate Professor Philip Ahring for providing me with valuable guidance in molecular biology and electrophysiology; Associate Professor Thomas Balle for his immense knowledge in molecular modelling; Dr Raja Viswas for the synthesis of various chemicals used in this study. I would also like to thank my colleagues and lab buddies (in no particular order): Vivian, Irene, Radhika, Zirong, Taima, Leonny, Jia, Bryan, Ting, Steve, Terry, Tim, Izumi, Ida and Maja for their friendship, support, shared knowledge and experiences over the years, which helped me stay sane through those hair-pulling moments. Last but not least, my heartfelt gratitude goes to my family, as none of this would have been possible without their support. They have been a constant source of love, concern, support and strength throughout all these years and I deeply appreciate their faith in me. Abstract GABAA receptors (GABAARs) are a class of physiologically- and therapeutically- important ligand-gated ion channels. -

Bi-Directional Acceleration of Alcohol Use and Opioid Use Disorder Wenfei Huang1,2, Erika P
Ashdin Publishing Journal of Drug and Alcohol Research ASHDIN Vol. 8 (2019), Article ID 236084, 14 pages publishing doi:10.4303/jdar/236084 Review Article Bi-directional Acceleration of Alcohol Use and Opioid Use Disorder Wenfei Huang1,2, Erika P. Penaherrera1, Danaika F. Desir1, Dominick L. Gamarro1, Jessica Cottrell1, Tinchun Chu1 and Sulie L. Chang1,2 * 1Department of Biological Sciences, Seton Hall University, South Orange, NJ, USA 2Institute of NeuroImmune Pharmacology, Seton Hall University, South Orange, NJ, USA Address correspondence to Sulie L. Chang, [email protected] Received 14 August 2019 ; Revised 11 October 2019; Accepted 18 October 2019 Copyright © 2019 Sulie L. Chang, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract (NIAAA) as drinking that brings a person’s blood alcohol Alcohol is the most widely used addictive substance. Severe alcohol concentration (BAC) to ≥ 0.08 g % after consumption of abuse is diagnosed as “alcohol use disorder” (AUD). A common and 5 or more drinks for men or 4 or more drinks for women harmful drinking pattern is binge drinking that elevates a person’s in about 2 hours [6]. Binge drinking is the most common blood alcohol concentration to ≥ 0.08%. Such drinking may be an and deadly pattern of excessive alcohol use in the United early indicator of AUD. Opioid misuse and dependence have become worldwide crises. Patterned consumption of various opioids can States [7-9], being associated with health risks such as develop into opioid use disorder (OUD). -

Gaba Receptors, Third Edition T He R Eceptors
THE GABA RECEPTORS, THIRD EDITION T HE R ECEPTORS KIM A. NEVE, SERIES EDITOR The GABA Receptors, Third Edition, EDITED BY S. J. Enna and Hanns Möhler, 2007 The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics, EDITED BY Bryan L. Roth, 2006 The Adrenergic Receptors: In the 21st Century, EDITED BY Dianne M. Perez, 2005 The Melanocortin Receptors, EDITED BY Roger D. Cone, 2000 The GABA Receptors, Second Edition, EDITED BY S. J. Enna and Norman G. Bowery, 1997 The Ionotropic Glutamate Receptors, EDITED BY Daniel T. Monaghan and Robert Wenthold, 1997 The Dopamine Receptors, EDITED BY Kim A. Neve and Rachael L. Neve, 1997 The Metabotropic Glutamate Receptors, EDITED BY P. Jeffrey Conn and Jitendra Patel, 1994 The Tachykinin Receptors, EDITED BY Stephen H. Buck, 1994 The Beta-Adrenergic Receptors, EDITED BY John P. Perkins, 1991 Adenosine and Adenosine Receptors, EDITED BY Michael Williams, 1990 The Muscarinic Receptors, EDITED BY Joan Heller Brown, 1989 The Serotonin Receptors, EDITED BY Elaine Sanders-Bush, 1988 The Alpha-2 Adrenergic Receptors, EDITED BY Lee Limbird, 1988 The Opiate Receptors, EDITED BY Gavril W. Pasternak, 1988 The GABA Receptors THIRD EDITION Edited by S. J. Enna Departments of Molecular and Integrative Physiology and of Pharmcology, Toxicology, and Therapeutics University of Kansas Kansas City, KS and Hanns Möhler Institute of Pharmacology University of Zurich Department of Chemistry and Applied Biosciecnes Swiss Federal Institute of Technology (ETH) and Collegium Helveticum Zurich, Switzerland © 2007 Humana Press Inc. 999 Riverview Drive, Suite 208 Totowa, New Jersey 07512 www.humanapress.com All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording, or otherwise without written permission from the Publisher. -

Competitive Antagonism of Housefly Γ-Aminobutyric Acid Receptors By
Available online at www.banglajol.info Bangladesh J. Sci. Ind. Res. 56(1), 9-16, 2021 competitive antagonists, which share the same orthosteric which disturb the GABA receptor functions. Gabazine (3) of insect pest control chemicals in future. oocytes were constantly perfused with SOS solution at 18 to our previous study for comparison (Rahman et al., 2014). housefly GABA receptors (Rahman et al., 2014). High Buckingham SD, Biggin PC, Sattelle BM, Brown LA and Johnston GAR (1996), GABAA receptor pharmacology, Ozoe Y (2013), γ-aminobutyrate- and glutamate-gated Sattelle DB, Lummis SCR, Wong JFH and Rauh JJ (1991), binding site with agonists, and ii) noncompetitive (Fig. 1) is a recognized competitive antagonist for 22 °C. GABA was dissolved in SOS for agonist assays and The inhibition percentage of gabazine (with 4-methoxy similarity (71.1%) between housefly GABA receptor Sattelle DB (2005), Insect GABA receptors: splicing, Pharmacol. Ther. 69: 173-198. DOI: 10.1016/0163- chloride channels as targets of insecticides, Adv. Insect Pharmacology of insect GABA receptors, Neurochem. Materials and methods antagonists, binding in the allosteric site in the channel mammalian GABAA receptors (Chambon et al. 1985, Ueno applied to the oocytes for 3 s with intervals of 60 s. group at 3-position of pyridazine ring) is presented here as amino acid sequence (GenBank accession AB177547) and editing, and targeting by antiparasitics and 7258(95)02043-8 Physiol. 44: 211-286. DOI: 10.1016/B978-0-12- Res. 16: 363-374. DOI: 10.1007/BF00966100 domain (Johnston, 1996). Competitive antagonists binding to et al. 1997). However, it was weak to moderately active Increasing concentrations of GABA were sequentially standard which exhibited very weak activity with only insecticides, Mol.