Gaba Receptors, Third Edition T He R Eceptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2017/0020892 A1 Thompson Et Al
US 20170020892A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0020892 A1 Thompson et al. (43) Pub. Date: Jan. 26, 2017 (54) USE OF NEGATIVE MODULATORS OF Related U.S. Application Data GABA RECEPTORS CONTAINING ALPHAS SUBUNITS AS FAST ACTING (60) Provisional application No. 61/972,446, filed on Mar. ANTDEPRESSANTS 31, 2014. (71) Applicant: University of Maryland, Baltimore, Publication Classification Baltimore, MD (US) (51) Int. Cl. A 6LX 3/557 (2006.01) (72) Inventors: Scott Thompson, Baltimore, MD (US); A6II 3/53 (2006.01) Mark D. Kvarta, Ellicott City, MD A6II 45/06 (2006.01) (US); Adam Van Dyke, Baltimore, MD (52) U.S. Cl. (US) CPC ........... A61 K3I/55.17 (2013.01); A61K 45/06 (2013.01); A61 K3I/53 (2013.01) (73) Assignee: University of Maryland, Baltimore, Baltimore, MD (US) (57) ABSTRACT Embodiments of the disclosure include methods and com (21) Appl. No.: 15/300,984 positions related to treatment of one or more medical conditions with one or more negative modulators of GABA (22) PCT Filed: Mar. 31, 2015 receptors. In specific embodiments, depression and/or Sui cidability is treated or ameliorated or prevented with one or (86) PCT No.: PCT/US2O15/023667 more negative modulators of GABA receptors, such as a S 371 (c)(1), partial inverse agonist of a GABA receptor comprising an (2) Date: Sep. 30, 2016 alpha5 subunit. Patent Application Publication Jan. 26, 2017. Sheet 1 of 12 US 2017/002O892 A1 ×1/ /|\ Patent Application Publication Jan. 26, 2017. Sheet 3 of 12 US 2017/002O892 A1 & Patent Application Publication Jan. -
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
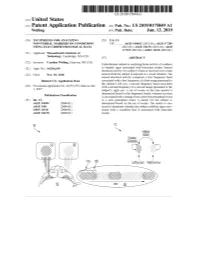
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Cognition and Steroidogenesis in the Rhesus Macaque
Cognition and Steroidogenesis in the Rhesus Macaque Krystina G Sorwell A DISSERTATION Presented to the Department of Behavioral Neuroscience and the Oregon Health & Science University School of Medicine in partial fulfillment of the requirements for the degree of Doctor of Philosophy November 2013 School of Medicine Oregon Health & Science University CERTIFICATE OF APPROVAL This is to certify that the PhD dissertation of Krystina Gerette Sorwell has been approved Henryk Urbanski Mentor/Advisor Steven Kohama Member Kathleen Grant Member Cynthia Bethea Member Deb Finn Member 1 For Lily 2 TABLE OF CONTENTS Acknowledgments ......................................................................................................................................................... 4 List of Figures and Tables ............................................................................................................................................. 7 List of Abbreviations ................................................................................................................................................... 10 Abstract........................................................................................................................................................................ 13 Introduction ................................................................................................................................................................. 15 Part A: Central steroidogenesis and cognition ............................................................................................................ -

The Human Carotid Body Expression of Oxygen Sensing and Signaling Genes of Relevance for Anesthesia
PERIOPERATIVE MEDICINE Anesthesiology 2010; 113:1270–9 Copyright © 2010, the American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins The Human Carotid Body Expression of Oxygen Sensing and Signaling Genes of Relevance for Anesthesia Malin Jonsson Fagerlund, M.D., Ph.D.,* Jessica Kåhlin, M.D.,† Anette Ebberyd, B.M.A.,‡ Gunnar Schulte, Ph.D.,§ Souren Mkrtchian, M.D., Ph.D.,ʈ Lars I. Eriksson, M.D., Ph.D., F.R.C.A.# Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/113/6/1270/252613/0000542-201012000-00011.pdf by guest on 28 September 2021 ABSTRACT with DNA microarrays, real-time polymerase chain reaction, Background: Hypoxia is a common cause of adverse events and immunohistochemistry. in the postoperative period, where respiratory depression due Results: We found gene expression of the oxygen-sensing ϩ to residual effects of drugs used in anesthesia is an important pathway, heme oxygenase 2, and the K channels TASK ϩ underlying factor. General anesthetics and neuromuscular (TWIK-related acid sensitive K channel)-1 and BK (large- blocking agents reduce the human ventilatory response to conductance potassium channel). In addition, we show the hypoxia. Although the carotid body (CB) is the major oxygen expression of critical receptor subunits such as ␥-aminobu- ␣  ␥ sensor in humans, critical oxygen sensing and signaling path- tyric acid A ( 2, 3, and 2), nicotinic acetylcholine recep- ␣ ␣  ways have been investigated only in animals so far. Thus, the tors ( 3, 7, and 2), purinoceptors (A2A and P2X2), and aim of this study was to characterize the expression of key the dopamine D2 receptor. genes and localization of their products involved in the hu- Conclusions: In unique samples of the human CB, we here man oxygen sensing and signaling pathways with a focus on demonstrate presence of critical proteins in the oxygen-sens- receptor systems and ion channels of relevance in anesthesia. -

Structural Basis for Potentiation by Alcohols and Anaesthetics in a Ligand-Gated Ion Channel
ARTICLE Received 10 Jul 2012 | Accepted 28 Feb 2013 | Published 16 Apr 2013 DOI: 10.1038/ncomms2682 Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel Ludovic Sauguet1,2,3,4,*, Rebecca J. Howard5,w,*, Laurie Malherbe1,2,3,4,UiS.Lee5, Pierre-Jean Corringer3,4, R. Adron Harris5 & Marc Delarue1,2 Ethanol alters nerve signalling by interacting with proteins in the central nervous system, particularly pentameric ligand-gated ion channels. A recent series of mutagenesis experi- ments on Gloeobacter violaceus ligand-gated ion channel, a prokaryotic member of this family, identified a single-site variant that is potentiated by pharmacologically relevant concentra- tions of ethanol. Here we determine crystal structures of the ethanol-sensitized variant in the absence and presence of ethanol and related modulators, which bind in a transmembrane cavity between channel subunits and may stabilize the open form of the channel. Structural and mutagenesis studies defined overlapping mechanisms of potentiation by alcohols and anaesthetics via the inter-subunit cavity. Furthermore, homology modelling show this cavity to be conserved in human ethanol-sensitive glycine and GABA(A) receptors, and to involve residues previously shown to influence alcohol and anaesthetic action on these proteins. These results suggest a common structural basis for ethanol potentiation of an important class of targets for neurological actions of ethanol. 1 Unite´ de Dynamique Structurale des Macromole´cules, Institut Pasteur, F-75015 Paris, France. 2 UMR 3258, Centre National de la Recherche Scientifique, F-75015 Paris, France. 3 Groupe Re´cepteurs-Canaux, Institut Pasteur, F-75015 Paris, France. 4 Unite´ de Recherche Associe´e 2182, Centre National de la Recherche Scientifique, F-75015 Paris, France. -

Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors
International Journal of Molecular Sciences Article Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors Hana Kubová 1,* , Zde ˇnkaBendová 2,3 , Simona Moravcová 2,3 , Dominika Paˇcesová 2,3, Luisa Rocha 4 and Pavel Mareš 1 1 Institute of Physiology, Academy of Sciences of the Czech Republic, 14220 Prague, Czech Republic; [email protected] 2 Faculty of Science, Charles University, 12800 Prague, Czech Republic; [email protected] (Z.B.); [email protected] (S.M.); [email protected] (D.P.) 3 National Institute of Mental Health, 25067 Klecany, Czech Republic 4 Pharmacobiology Department, Center of Research and Advanced Studies, Mexico City 14330, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +420-2-4106-2565 Received: 31 March 2020; Accepted: 28 April 2020; Published: 30 April 2020 Abstract: Benzodiazepines (BZDs) are widely used in patients of all ages. Unlike adults, neonatal animals treated with BZDs exhibit a variety of behavioral deficits later in life; however, the mechanisms underlying these deficits are poorly understood. This study aims to examine whether administration of clonazepam (CZP; 1 mg/kg/day) in 7–11-day-old rats affects Gama aminobutyric acid (GABA)ergic receptors in both the short and long terms. Using RT-PCR and quantitative autoradiography, we examined the expression of the selected GABAA receptor subunits (α1, α2, α4, γ2, and δ) and the GABAB B2 subunit, and GABAA, benzodiazepine, and GABAB receptor binding 48 h, 1 week, and 2 months after treatment discontinuation. Within one week after CZP cessation, the expression of the α2 subunit was upregulated, whereas that of the δ subunit was downregulated in both the hippocampus and cortex. -

Gene Expression Profile in Different Age Groups and Its Association With
cells Article Gene Expression Profile in Different Age Groups and Its Association with Cognitive Function in Healthy Malay Adults in Malaysia Nur Fathiah Abdul Sani 1 , Ahmad Imran Zaydi Amir Hamzah 1, Zulzikry Hafiz Abu Bakar 1 , Yasmin Anum Mohd Yusof 2, Suzana Makpol 1 , Wan Zurinah Wan Ngah 1 and Hanafi Ahmad Damanhuri 1,* 1 Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Center, Jalan Yaacob Latif, Cheras, Kuala Lumpur 56000, Malaysia; [email protected] (N.F.A.S.); [email protected] (A.I.Z.A.H.); zulzikryhafi[email protected] (Z.H.A.B.); [email protected] (S.M.); [email protected] (W.Z.W.N.) 2 Faculty of Medicine and Defence Health, National Defence University of Malaysia, Kem Sungai Besi, Kuala Lumpur 57000, Malaysia; [email protected] * Correspondence: hanafi[email protected] Abstract: The mechanism of cognitive aging at the molecular level is complex and not well under- stood. Growing evidence suggests that cognitive differences might also be caused by ethnicity. Thus, this study aims to determine the gene expression changes associated with age-related cognitive decline among Malay adults in Malaysia. A cross-sectional study was conducted on 160 healthy Malay subjects, aged between 28 and 79, and recruited around Selangor and Klang Valley, Malaysia. Citation: Abdul Sani, N.F.; Amir Gene expression analysis was performed using a HumanHT-12v4.0 Expression BeadChip microarray Hamzah, A.I.Z.; Abu Bakar, Z.H.; kit. The top 20 differentially expressed genes at p < 0.05 and fold change (FC) = 1.2 showed that Mohd Yusof, Y.A.; Makpol, S.; Wan PAFAH1B3, HIST1H1E, KCNA3, TM7SF2, RGS1, and TGFBRAP1 were regulated with increased Ngah, W.Z.; Damanhuri, H.A. -

The Role of GABRA2 in Risk for Conduct Disorder and Alcohol and Drug Dependence Across Developmental Stages
Behavior Genetics, Vol. 36, No. 4, July 2006 (Ó 2006) DOI: 10.1007/s10519-005-9041-8 The Role of GABRA2 in Risk for Conduct Disorder and Alcohol and Drug Dependence across Developmental Stages Danielle M. Dick,1,9 Laura Bierut,1 Anthony Hinrichs,1 Louis Fox,1 Kathleen K. Bucholz,1 John Kramer,2 Samuel Kuperman,2 Victor Hesselbrock,3 Marc Schuckit,4 Laura Almasy,5 Jay Tischfield,6 Bernice Porjesz,7 Henri Begleiter,7 John Nurnberger Jr.,8 Xiaoling Xuei,8 Howard J. Edenberg,8 and Tatiana Foroud8 Received Apr. 28 2005—Final Dec. 22 2005 We use findings from the behavior genetics literature about how genetic factors (latently) influence alcohol dependence and related disorders to develop and test hypotheses about the risk associated with a specific gene, GABRA2, across different developmental stages. This gene has previously been associated with adult alcohol dependence in the Collaborative Study of the Genetics of Alcoholism (COGA) sample [Edenberg, H. J., Dick, D. M., Xuei, X., Tian, H., Almasy, L., Bauer, L. O., Crowe, R., Goate, A., Hesselbrock, V., Jones, K. A., Kwon, J., Li, T. K., Nurnberger Jr., J. I., OÕConnor, S. J., Reich, T., Rice, J., Schuckit, M., Porjesz, B., Foroud, T., and Begleiter, H. (2004). Am. J. Hum. Genet. 74:705–714] and other studies [Covault, J., Gelernter, J., Hesselbrock, V., Nellissery, M., and Kranzler, H. R. (2004). Am. J. Med. Genet. B Neuropsychiatr. Genet. 129B:104–109; Lappalainen, J., Krupitsky, E., Remizov, M., Pchelina, S., Taraskina, A., Zvartau, E., Somberg, L. K., Covault, J., Kranzler, H. R., Krystal, J., and Gelernter, J. -

Gabaergic Signaling Linked to Autophagy Enhances Host Protection Against Intracellular Bacterial Infections
ARTICLE DOI: 10.1038/s41467-018-06487-5 OPEN GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections Jin Kyung Kim1,2,3, Yi Sak Kim1,2,3, Hye-Mi Lee1,3, Hyo Sun Jin4, Chiranjivi Neupane 2,5, Sup Kim1,2,3, Sang-Hee Lee6, Jung-Joon Min7, Miwa Sasai8, Jae-Ho Jeong 9,10, Seong-Kyu Choe11, Jin-Man Kim12, Masahiro Yamamoto8, Hyon E. Choy 9,10, Jin Bong Park 2,5 & Eun-Kyeong Jo1,2,3 1234567890():,; Gamma-aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the brain; however, the roles of GABA in antimicrobial host defenses are largely unknown. Here we demonstrate that GABAergic activation enhances antimicrobial responses against intracel- lular bacterial infection. Intracellular bacterial infection decreases GABA levels in vitro in macrophages and in vivo in sera. Treatment of macrophages with GABA or GABAergic drugs promotes autophagy activation, enhances phagosomal maturation and antimicrobial responses against mycobacterial infection. In macrophages, the GABAergic defense is mediated via macrophage type A GABA receptor (GABAAR), intracellular calcium release, and the GABA type A receptor-associated protein-like 1 (GABARAPL1; an Atg8 homolog). Finally, GABAergic inhibition increases bacterial loads in mice and zebrafish in vivo, sug- gesting that the GABAergic defense plays an essential function in metazoan host defenses. Our study identified a previously unappreciated role for GABAergic signaling in linking antibacterial autophagy to enhance host innate defense against intracellular bacterial infection. 1 Department of Microbiology, Chungnam National University School of Medicine, Daejeon 35015, Korea. 2 Department of Medical Science, Chungnam National University School of Medicine, Daejeon 35015, Korea. -

Characterisation of GABAA Receptors and Cation-Chloride Cotransporters in the Uterus and Their Role in Pre-Term Labour
Characterisation of GABAA receptors and cation-chloride cotransporters in the uterus and their role in pre-term labour Melissa Linda Sutherland December 2017 Supervisors: Dr. Amy V. Poole, Dr. Jennifer A. Fraser, Dr. Claire Garden. A thesis submitted in partial fulfilment of the requirements of Edinburgh Napier University, for the award of Master by Research Declaration It is hereby declared that this thesis is the result of the author’s original research. It has been composed by the author and has not been previously submitted for examination, which has led to the award of a degree or professional qualification. Signed: Date: Contents page Abbreviations .............................................................................................. 1 Acknowledgements ................................................................................... 3 Abstract ......................................................................................................... 4 CHAPTER 1. Introduction ......................................................................... 5 1.1-aminobutyric acid (GABA) .............................................................. 5 1.2 GABA receptor structure and function .......................................... 5 Figure 1.1 Schematic diagram of the GABAA subunit and receptor ......................................................................................................... 6 1.3 GABAARs role in development central nervous system .......................................................................................................... -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties.