Minerals-10-00395-V2.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

The Importance of Minerals in Coal As the Hosts of Chemical Elements: a Review
The importance of minerals in coal as the hosts of chemical elements: A review Robert B. Finkelmana,b, Shifeng Daia,c,*, David Frenchd a State Key Laboratory of Coal Resources and Safe Mining, China University of Mining and Technology, China b University of Texas at Dallas, Richardson, TX 75080, USA c College of Geoscience and Survey Engineering, China University of Mining and Technology (Beijing), Beijing 100083, China d PANGEA Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia *, Corresponding author: [email protected]; [email protected] Abstract Coal is a complex geologic material composed mainly of organic matter and mineral matter, the latter including minerals, poorly crystalline mineraloids, and elements associated with non- mineral inorganics. Among mineral matter, minerals play the most significant role in affecting the utilization of coal, although, in low rank coals, the non-mineral elements may also be significant. Minerals in coal are often regarded as a nuisance being responsible for most of the problems arising during coal utilization, but the minerals are also seen as a potentially valuable source of critical metals and may also, in some cases, have a beneficial effect in coal gasification and liquefaction. With a few exceptions, minerals are the major hosts of the vast majority of elements present in coal. In this review paper, we list more than 200 minerals that have been identified in coal and its low temperature ash, although the validity of some of these minerals has not been confirmed. Base on chemical compositions, minerals found in coal can be classified into silicate, sulfide and selenide, phosphate, carbonate, sulfate, oxide and hydroxide, and others. -

ATOMIC ENERGY £2?A L'energie ATOMIQUE of CANADA LIMITED \Fijt DU CANADA,LIMITEE
w *£.''• 7 / J, i. AECL-7792 ATOMIC ENERGY £2?A L'ENERGIE ATOMIQUE OF CANADA LIMITED \fijT DU CANADA,LIMITEE THE CONVERSION OF SMECTITE TO ILLITE IN HYDROTHERMAL SYSTEMS: A LITERATURE REVIEW LA CONVERSION OE LA SMECTITE EN ILLITE DANS LES SYSTEMES HYDROTHERMIQUE: EXAMEN DES TRAVAUX PUBLIES R.M. Johnston Whiteshell Nuclear Research Etabiissement de recherches Establishment i>ucleaires de Whiteshel! Pinawa, Manitoba ROE 1LO June 1983 juin Copyright © Atomic Energy of Canada Limited, 1983 ATOMIC ENERGY OF CANADA LIMITED THE CONVERSION OF SMECTITE TO ILLITE IN HYDROTHERMAL SYSTEMS: A LITERATURE REVIEW by R.M. Johnston Whiteshell Nuclear "esearch Establishment Pinawa, Manitoba ROE 1L0 1983 June AECL-7792 LA CONVERSION DE LA SMECTITE EN ILLITE DANS LES SYSTÈMES HYDROTHERMIQUE: EXAMEN DES TRAVAUX PUBLIÉS par R.M. Johnston RESUME Dans les systèmes schisteux diagénétiques naturels, la smectite se transforme en illlte et en illite-smectite à couche mixte en moins d'un mil- lion d'années, en présence de températures se situant entre 75°C et 200°C. De ce fait, certaines questions se posent quant à la stabilité des tampons de bentonite à base de smectite par rapports aux conditions propres â une enceinte d'évacuation nucléaire• Les données expérimentales et géologiques obtenues indiquent que la réaction dépend de la disponibilité des K*" et que le taux de réaction des systèmes pauvres en K* (tels que les enceintes d'é- vacuation) peut être bien inférieur â ce que l'on observe dans le schiste. La présence de Na+, de Ca2+ et de Mg2+ dans le système ralentie la réaction et peut même l'arrêter complètement à des températures inférieures. -

Download the Scanned
BOOK REVIEWS THE INTERPRETATION OF X-RAY DIFFRACTION PHOTOGRAPHS, bv N. M. F. HrNnY, H. Lrrson arvl W. A. Woosrnn, MacMillan and Co., London, 1951, ix*258 pages, price 42 shillings. (American price $8.50, obtained from D. Van Nostrand Co., Inc., New York, N. Y.) This book is intended "to help students and research workers to understand the theory and practice of the interpretation of r-ray difiraction photographs'" The discussion is carried up to the subject of crystal-structure determination but does not include more than passing reference to space groups and their recognition by means of *-ray diffraction. Had a chapter on space groups been included, and it would have required no more space than that devoted to Laue photographs in the present volume, all materials needed pre- paratory to crystal structure work would have been brought together. The omission is the more regrettable because the authors state that "a sufficient number of tables has been included to facilitate calculations without the necessity of a reference book." Many who do not embark upon crystal structure work do attempt the determination of space groups and a statement of the space group, now considered part of a complete mineral description, is to be found in several recently published mineralogical reference books. What seems to this reviewer the pedagogical excellence of Henry, Lipson and Wooster's book is probably due in part to the fact that the authors have been associated with the very thorough course in crystallography which has been ofiered at the University of Cambridge for many years. -

Brammallite (Sodium-Iuite),. a New Mineral from Llandebie, South Wales
304 Brammallite (sodium-iUite),. a new mineral from Llandebie, South Wales. By F. A. BANNISTER,M.A. Deputy Keeper, Mineral Department, British Museum. [Read June 4, 1942.] URING a study of the petrography and mineral constituents separated from D various shales overlying the coal-measures of South Wales, Dr. Alfred Brammull noticed on several hand-specimens from Llandebie a white infilling to fissures or a coating on slickensided surfaces which could be readily detached from the matrix. Chemical examination showed that this coating, unlike the shales themselves, contained more sodium than potassium. The present note gives an account of subsequent chemical, X-ray, and optical work , which confirms Dr. Brammall's suggestion that the white incrustation contains a new sodium- rich mineral allied to mica. It is not, I hope, an unfitting tribute to his work in this field as well as to his interest in recent advances in mineralogy that I have named this mineral brammallite. A short account of its nature and relationship to illite, which is the chief constituent of the shale itself (see preceding paper, p. 297), will now be given. Examination under the binocular microscope revealed that most of the in- crustation consists of a soft fibrous mineral; and, when this is detached with a knife, small compact tufts of elongated plates about ram. long, reminiscent of small fragments of satin-spar, can be distinguished and isolated for optical and X-ray work. Not sufficient material, however, was available for a micro- chemical analysis. Dr. M. H. Hey carried out an alkali determination on 15 mg. -

Book Reviews
767 The Canadian Mineralogist Vol. 38, pp. 767-776 (2000) BOOK REVIEWS Crystal Habits of Minerals, by Ivan Kostov and Ruslan Chapter 6, Crystal habits of Important Minerals I. Kostov, 1999. 415 pages, hardbound. Prof. Marin (248 pages), is divided into: (1) native elements Drinov Academic Publishing House & Pensoft Publish- (15 pages), (2) sulphide and related minerals (44 pages), ers, Sofia, Bulgaria. Full address: Akad. G. Bonchev (3) oxide and hydroxide minerals (26 pages), (4) halide Str., Bl6, 1113 Sofia, Bulgaria. $65 + $7 shipping and minerals (9 pages), (5) silicate minerals (91 pages), (6) handling (US). Credit card payment is acceptable. borate minerals (6 pages), (7) phosphate and related minerals (14 pages), (8) molybdate and tungstate min- Every now and then, a book is published and my erals (6 pages), (9) sulphate minerals (14 pages), (10) reaction is “Why wasn’t this published long ago?” Crys- carbonate minerals (19 pages), (11) chromate, nitrate tal Habits of Minerals is such a book. The Bulgarian and iodate minerals (2 pages) and (12) organic minerals father and son who put this book together are to be con- (2 pages). gratulated for compiling a massive amount of data on this fascinating subject. In doing this, they also acted as Chapter 7, Applied Crystal Morphology (16 pages), a “bridge” between Bulgarian/Russian literature and that consists of (1) General Notes, (2) Crystal Habits Ap- of the western world. The text is well written and does plied to Geosciences and Crystal Habits Applied to In- not suffer from the failings of many books written by dustries. -

Nomenclature of the Micas
Mineralogical Magazine, April 1999, Vol. 63(2), pp. 267-279 Nomenclature of the micas M. RIEDER (CHAIRMAN) Department of Geochemistry, Mineralogy and Mineral Resources, Charles University, Albertov 6, 12843 Praha 2, Czech Republic G. CAVAZZINI Dipartimento di Mineralogia e Petrologia, Universith di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy Yu. S. D'YAKONOV VSEGEI, Srednii pr., 74, 199 026 Sankt-Peterburg, Russia W. m. FRANK-KAMENETSKII* G. GOTTARDIt S. GUGGENHEIM Department of Geological Sciences, University of Illinois at Chicago, 845 West Taylor St., Chicago, IL 60607-7059, USA P. V. KOVAL' Institut geokhimii SO AN Rossii, ul. Favorskogo la, Irkutsk - 33, Russia 664 033 G. MOLLER Institut fiir Mineralogie und Mineralische Rohstoffe, Technische Universit/it Clausthal, Postfach 1253, D-38670 Clausthal-Zellerfeld, Germany A. M, R. NEIVA Departamento de Ci6ncias da Terra, Universidade de Coimbra, Apartado 3014, 3049 Coimbra CODEX, Portugal E. W. RADOSLOVICH$ J.-L. ROBERT Centre de Recherche sur la Synth6se et la Chimie des Min6raux, C.N.R.S., 1A, Rue de la F6rollerie, 45071 Od6ans CEDEX 2, France F. P. SASSI Dipartimento di Mineralogia e Petrologia, Universit~t di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy H. TAKEDA Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino City, Chiba 275, Japan Z. WEISS Central Analytical Laboratory, Technical University of Mining and Metallurgy, T/'. 17.1istopadu, 708 33 Ostrava- Poruba, Czech Republic AND D. R. WONESw * Russia; died 1994 t Italy; died 1988 * Australia; resigned 1986 wUSA; died 1984 1999 The Mineralogical Society M. RIEDER ETAL. ABSTRACT I I End-members and species defined with permissible ranges of composition are presented for the true micas, the brittle micas, and the interlayer-deficient micas. -

The Classification and Nomenclature of Clay Minerals
THE CLASSIFICATION AND NOMENCLATURE OF CLAY MINERALS By R. C. MACKENZIE The Macaulay Institute for Soil Research, Craigiebuckler, Aberdeen [Received 21st April, 1959] ABSTRACT A report is given of the decisions reached at a meeting on the classifica- tion and nomenclature of clay minerals held under the auspices of Comit~ International pour l'Etude des Argiles at Brussels in July 1958. Tables of recently-proposed classification systems are included and some com- ments made on problems requiring agreement. The increasing interest in the classification and nomenclature of clay minerals over the last decade or so may be attributed largely to the development of investigational methods which enable a much more precise characterization of fine-grained minerals than was previously possible. In addition, however, the absence of any hard-and-fast rules as to new mineral nomenclature has led to a multiplicity of names through "new" minerals being described on the flimsiest of evidence, without regard as to whether they might be considered varieties of an already-established entity or indeed with- out, in many instances, adequate evidence of homogeneity. The resulting confusion is considerable, and the stage has now been reached when international agreement upon the main features of classification and nomenclature ought to be obtained. In an attempt to clarify the position representatives often countries met, under the" auspices of C.I.P.E.A., during the Journ6es Inter- nationales d'Etude des Argiles in Brussels in July, 1958.* Several decisions of interest were made at this meeting. (a) The following definition was adopted, subject to confirmation at the next meeting: Crystalline day minerals are hydrated silicates with layer or chain lattices consisting of sheets of silica tetrahedra arranged in hexagonal form condensed with octahedral layers; they are usually of small particle size. -

The Influence of Individual Clay Minerals on Formation Damage of Reservoir Sandstones: a Critical Review with Some New Insights
Clay Minerals, (2014) 49, 147–164 OPEN ACCESS The influence of individual clay minerals on formation damage of reservoir sandstones: a critical review with some new insights 1, 2 2 M. J. WILSON *, L. WILSON AND I. PATEY 1 James Hutton Institute, Aberdeen, UK, and 2 Corex(UK)Ltd,HoweMossDrive,Aberdeen,UK (Received 29 June 2012; revised 26 July 2012; Editor: Harry Shaw) ABSTRACT: The influence of individual clay minerals on formation damage of reservoir sandstones is reviewed, mainly through the mechanism of fine particle dispersion and migration leading to the accumulation and blockage of pore throats and significant reduction of permeability. The minerals discussed belong to the smectite, kaolinite, illite and chlorite groups respectively. These minerals usually occur in an aggregate form in reservoir sandstones and the physicochemical properties of these aggregates are reviewed in order to reach a better understanding of the factors that lead to their dispersion in aqueous pore fluids. Particularly significant properties include the surface charge on both basal and edge faces of the clay minerals and how this varies with pH, external surface area of both swelling and non-swelling clays, porosity and pore size distribution in the micro- and meso-pore size range and overall aggregate morphology. For non-swelling clays, and perhaps even for swelling clays, dispersion is thought to be initiated at the micro- or meso-pore level, where the interaction between the pore solution and the charged clay surfaces exposed on adjacent sides of slit- or wedge-shaped pores brings about expansion of the diffuse double electric layer (DDL) and an increase in hydration pressure. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -
Durham E-Theses
Durham E-Theses The response of coarse and ne coal-mine discards under controlled load triaxial testing Kennedy, George W. How to cite: Kennedy, George W. (1977) The response of coarse and ne coal-mine discards under controlled load triaxial testing, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/8986/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 THE RESPONSE OF COARSE AND FINE COAL-MINE DISCARDS UNDER CONTROLLED LOAD THIAXIAL TESTING by George' W. Kennedy being a Thesis submitted for the Degree of Master of Science in the University of Durham. September 1977 The copyright of this thesis rests with the author. No quotation from it should be published without '::J his prior written consent and information derived ^ "" from it should be acknowledged. (i:): ABSTRACT The aim of the project was to investigate the behaviour of coar.se and fine colliery discards with respect to liquefaction potential, using controlled load triaxial testing. -
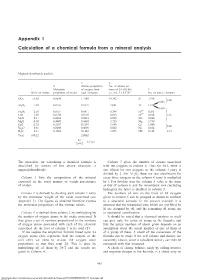
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜.