Characterization of Algerian Honey from Tiaret Region and Immunoassay Study of Its Immunomodulatory Effect in BALB/C Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal Officiel Algérie
N° 64 Dimanche 19 Safar 1440 57ème ANNEE Correspondant au 28 octobre 2018 JJOOUURRNNAALL OOFFFFIICCIIEELL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANÇAISE) Algérie ETRANGER DIRECTION ET REDACTION Tunisie SECRETARIAT GENERAL ABONNEMENT Maroc (Pays autres DU GOUVERNEMENT ANNUEL Libye que le Maghreb) WWW.JORADP.DZ Mauritanie Abonnement et publicité: IMPRIMERIE OFFICIELLE 1 An 1 An Les Vergers, Bir-Mourad Raïs, BP 376 ALGER-GARE Tél : 021.54.35..06 à 09 Edition originale.................................. 1090,00 D.A 2675,00 D.A 021.65.64.63 Fax : 021.54.35.12 Edition originale et sa traduction...... 2180,00 D.A 5350,00 D.A C.C.P. 3200-50 ALGER TELEX : 65 180 IMPOF DZ (Frais d'expédition en sus) BADR : 060.300.0007 68/KG ETRANGER : (Compte devises) BADR : 060.320.0600 12 Edition originale, le numéro : 14,00 dinars. Edition originale et sa traduction, le numéro : 28,00 dinars. Numéros des années antérieures : suivant barème. Les tables sont fournies gratuitement aux abonnés. Prière de joindre la dernière bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne 19 Safar 1440 2 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 64 28 octobre 2018 SOMMAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX Décret présidentiel n° 18-262 du 6 Safar 1440 correspondant au 15 octobre 2018 portant ratification du protocole de coopération entre le Gouvernement de la République algérienne démocratique et populaire et le Gouvernement de la République du Mali sur l'échange de connaissances et d'expériences dans le domaine juridique et judiciaire, signé à Alger, le 15 mai 2017............... -

Rapport Sur Les Priorités Et La Planification Année 2021
République Algérienne Démocratique et Populaire Ministère des Ressources en Eau Rapport sur les priorités et la planification Année 2021 Volume 2 Octobre/ 2020 Table des matières Contenu Section 1. Message du ministre .......................................................................................4 1.1 Message du ministre ...................................................................................................4 1.2 Déclaration du Secrétaire Général ..............................................................................5 Section 2. Au sujet du portefeuille...................................................................................6 2.1 La mission ...................................................................................................................6 - Production de l’eau domestique, industrielle et agricole, y compris la production et l’utilisation de l’eau de mer dessalée, de l’eau saumâtre et des eaux usées Épurées ;6 2.2 Le ministère ................................................................................................................7 2.3 Fiche Portefeuille ........................................................................................................9 Gestionnaire responsable : Ministre des Ressources en Eau ...............................................9 2.4 Planification des activités pour l’année 2021 ...........................................................11 Section 3. Planification détaillée du programme 01 ......................................................12 -

Journal Officiel = De La Republique Algerienne Democratique Et Populaire Conventions Et Accords Internationaux - Lois Et Decrets
No 22 ~ Mercredi 14 Moharram 1421 ~ . 39 ANNEE correspondant au 19 avril 2000 Pee nls 43 Ub! sess Sbykelig bte é yr celyly S\,\n JOURNAL OFFICIEL = DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX - LOIS ET DECRETS. ARRETES, DECISIONS, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANCAISE) Algérie ; ER DIRECTION ET REDACTION: Tunisie ETRANGER SECRETARIAT GENERAL ABONNEMENT Maroc (Pays autres DU GOUVERNEMENT ANNUEL Libyeye que le Maghreb) ” , Mauritanie Abonnement et publicité: : IMPRIMERIE OFFICIELLE 1 An- 1 An 7,9 et 13 Av. A. Benbarek-ALGER Tél: 65.18.15 a 17 - C.C.P. 3200-50 | Edition originale.....ccccsesseeees 856,00 D.A| 2140,00 D.A _ ALGER Télex: 65 180 IMPOF DZ . BADR: 060.300.0007 68/KG Edition originale et sa traduction}1712,00 D.A|. .4280,00 D.A ETRANGER: (Compte devises): (Frais d'expédition en sus) BADR: 060.320.0600 12 Edition originale, le numéro : 10,00 dinars. Edition originale et sa traduction, le numéro : 20,00 dinars. Numéros des années antérieures : suivant baréme. Les tables sont fournies gratuitement aux abonnés. Priére de joindre la derniére bande pour renouvellement, réclamation, et changement d'adresse. Tarif des insertions : 60,00 dinars la ligne. JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 22.14 Moharram 1421 19 avril 2000 SOMMAIRE | | ; ARRETES, DECISIONS ET AVIS | MINISTERE DES FINANCES Arrété du 13 Ramadhan 1420 correspondant au 21 décembre 1999 modifiant et complétant l'arrété du 26 Rajab 1416 -correspondant au 19 décembre 1995 portant création des inspections des impéts dans les wilayas relevant de la _,direction régionale des imp6ts de Chlef... -

Livraison De Nouveaux Établissements Scolaires
L’Algérie profonde / Ouest Secteur de l’Éducation nationale à Tiaret Livraison de nouveaux établissements scolaires © D. R. Quatre groupes scolaires, implantés à Tiaret (2), Dahmouni et Sougueur, quatre CEM à Tiaret, Takhemaret, Sougueur et Oued Lili trois lycées à Tiaret, Dahmouni et Chehaima ainsi que 94 classes en extension au profit du cycle primaire seront livrés à l’occasion de la nouvelle rentrée scolaire à travers le territoire de la wilaya. Selon le directeur de l’éducation nationale, Abdelkader Madani, ces nouveaux établissements ont déjà reçu leurs équipements et seront opérationnels dès la rentrée scolaire prévue pour le 21 du mois en cours. Ces nouvelles infrastructures viennent renforcer celles déjà existantes, et qui se résument à 541 écoles primaires, 151 CEM et 62 lycées réparties à travers la wilaya de Tiaret. Deux autres lycées, implantés à Sidi Abdelghani et Sebaine, seront livrés en octobre prochain. Les nouvelles réalisations ont consommé une enveloppe financière de 260 milliards de centimes. Toutefois, d’autres mesures d’accompagnement seront aussi livrées, notamment au niveau des zones déshéritées, à savoir l’ouverture d’une dizaine de cantines scolaires au niveau des localités déshéritées comme Sidi Hosni, Sidi Ali Mellal, Zmalet Émir-Abdelkader, Aïn Dzarit, Bouchekif, Sidi Abderahmane et Sidi Bakhti. Néanmoins, en prévision de la saison hivernale, les responsables concernés ont tablé sur un programme conséquent lié au système de chauffage, notamment au niveau des zones d’ombre où la consommation du mazout est totalement éradiquée pour être remplacée par le gaz de ville ou le gaz propane pour les rares localités non desservies par le gaz naturel. -

Aba Nombre Circonscriptions Électoralcs Et Composition En Communes De Siéges & Pourvoir
25ame ANNEE. — N° 44 Mercredi 29 octobre 1986 Ay\j SI AS gal ABAN bic SeMo, ObVel , - TUNIGIE ABONNEMENT ANNUEL ‘ALGERIE MAROC ETRANGER DIRECTION ET REDACTION: MAURITANIE SECRETARIAT GENERAL Abonnements et publicité : Edition originale .. .. .. .. .. 100 D.A. 150 DA. Edition originale IMPRIMERIE OFFICIELLE et satraduction........ .. 200 D.A. 300 DA. 7 9 et 13 Av. A. Benbarek — ALGER (frais d'expédition | tg}, ; 65-18-15 a 17 — C.C.P. 3200-50 ALGER en sus) Edition originale, le numéro : 2,50 dinars ; Edition originale et sa traduction, le numéro : 5 dinars. — Numéros des années antérleures : suivant baréme. Les tables sont fourntes gratul »ment aux abonnés. Priére dé joindre les derniéres bandes . pour renouveliement et réclamation. Changement d'adresse : ajouter 3 dinars. Tarif des insertions : 20 dinars la ligne JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE CONVENTIONS ET ACCORDS INTERNATIONAUX LOIS, ORDONNANCES ET DECRETS ARRETES, DECISIONS, CIRCULAIRES, AVIS, COMMUNICATIONS ET ANNONCES (TRADUCTION FRANGAISE) SOMMAIRE DECRETS des ceuvres sociales au ministére de fa protection sociale, p. 1230. Décret n° 86-265 du 28 octobre 1986 déterminant les circonscriptions électorales et le nombre de Décret du 30 septembre 1986 mettant fin aux siéges & pourvoir pour l’élection a l’Assemblée fonctions du directeur des constructions au populaire nationale, p. 1217. , ministére de la formation professionnelle et du travail, p. 1230. DECISIONS INDIVIDUELLES Décret du 30 septembre 1986 mettant fin aux fonctions du directeur général da la planification Décret du 30 septembre 1986 mettant fin aux et de. la gestion industrielle au ministére de fonctions du directeur de la sécurité sociale et lindustrie lourde,.p. -

Th. Nasrallah
République Algérienne Démocratique et Populaire Ministère de l’Enseignement Supérieur et de la Recherche Scientifique Ecole Nationale Supérieure d’Agronomie El Harrach Thèse En vue de l’obtention du diplôme Doctorat En sciences agronomiques THEME Caractérisation de la variabilité morphologique de 21 provenances algériennes de chêne vert (Quercus rotundifolia Lam.), évaluation de leur adaptation écologique dans la région semi aride de Saïda. Présenté par : NASRALLAH Yahia. Magister en Agronomie, Spécialité Foresterie Soutenue publiquement le 07/04/2014 Devant le jury composé de: ABDELKRIM Hacène Président : Professeur (ENSA) KHELIFI Lakhdar Directeur de thèse : Professeur (ENSA) DERRIDJ Arezki Examinateur : Professeur (UMMTO) NEDJAHI Abdellah Examinateur : Directeur de Recherche (INRF ) Remerciements Au terme de ce travail, il m'est agréable de remercier vivement tous ceux qui ont contribués à sa réalisation. Je dois remercier particulièrement : Monsieur KHELIFI Lakhdar, Professeur à l'Ecole Nationale Supérieure Agronomique d’El Harrach-Alger, pour avoir accepté de diriger cette thèse et qui par sa compétence et sa patience a orienté mon travail, son aide a été aussi précieuse qu’utile. Je lui adresse mes vifs remerciements et ma reconnaissance. Monsieur ABDELKRIM Hacene, professeur à l'Ecole Nationale Supérieure Agronomique d’El Harrach-Alger. Je le remercie beaucoup pour avoir accepté de présider ce jury. Messieurs AREZKI Derridj et NEDJAHI Abdellah respectivement professeur à l’Université de Tizi Ouzou et Directeur Général à l’INRF, d'avoir bien voulus me faire l'honneur de juger ce travail. Je dois exprimer ma gratitude aussi au Dr MORSLI et LELLOUCHI Med du département de foresterie de l’ENSA, au Dr DUCCOSSO A et au Pr GERBER.S de l’INRA de Bordeaux, au Dr I CHUINE et Dr F. -

Communes Pauvres : Territoires, Populations Et Capacités D'action
REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE Ministère de la Solidarité Nationale, de la Famille et de la Communauté Nationale à l’Etranger Etude d’affinement de la carte de la pauvreté de 2000 Communes pauvres : territoires, populations et capacités d’action Rapport de synthèse Mars 2006 Réalisé en coopération avec le Programme des Nations Unies pour le Développement PN UD Par l’Agence Nationale d’Aménagement du Territoire REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE Ministère de la Solidarité Nationale, de la Famille et de la Communauté Nationale à l’Etranger Etude d’affinement de la carte de la pauvreté de 2000 Communes pauvres : territoires, populations et capacités d’action Rapport de synthèse Mars 2006 Réalisé en coopération avec le Programme des Nations Unies pour le Développement PN UD Par Agence Nationale d’Aménagement du Territoire Avant propos La conférence nationale organisée à ALGER, au mois d’Octobre 2000, dans les réalités nationales et de la traduire en termes spatiaux, d’équi- autour de la lutte contre la pauvreté et l’exclusion, à l’initiative de Son libre régional, afin d’éviter les pièges de l’approche bureaucratique en Excellence, Le Président de la République, Monsieur Abdelaziz termes de moyenne nationale, approche susceptible de voiler les dé- Bouteflika, première du genre, a illustré par son impact informatif séquilibres locaux. et sa portée multidimensionnelle toute la volonté politique à réduire la fracture sociale induite par tant d’écarts provoqués par la tragédie L’approfondissement de certaines de -

12 19 Dhou El Kaada 1434 25 Septembre 2013 JOURNAL
JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 47 19 Dhou El Kaada 1434 12 25 septembre 2013 Ressort territorial de la conservation Wilaya Designation de la conservation Daira Commune TLEMCEN Tlemcen Tlemcen Chetouane Chetouane, Ain Fezza, Amieur MANSOURAH Mansourah Mansourah, Terny Beni Hediel, Ain Ghoraba, Beni Mester Hennaya Hennaya, Zenata, Ouled Ryah SEBDOU Sebdou Sebdou, El Aricha, El Gor Beni Snous Beni Snous, Beni Bahdel, Azail Sidi Djillali Sidi Djillali, El Bouihi REMCHI Remchi Remchi, Ain Youcef, Beni Ouarsous, Sebaa Chioukh, El Fehoul Honaine Honaine, Beni Khaled, TLEMCEN Ouled Mimoun Ouled Mimoun, Beni Semiel, Oued Lakhdar OULED MIMOUN Ain Tallout Ain Tallout, Ain Nahala Bensekrane Bensekrane, Sidi Abdelli Ghazaouet Ghazaouet, Tianet, Dar Yaghmoracen, Souahlia GHAZAOUET Marsa Ben M'hidi Marsa Ben M'hidi, M'cirda Fouaga Bab El Assa Bab El Assa, Souk Thlata, Souani Nedroma Nedroma, Djebala Maghnia Maghnia, Hammam Bougherara Fellaoucene Fellaoucene, Ain Kebira, Ain Fetah MAGHNIA Sabra Sabra, Bouhlou Beni Boussaid Beni Boussaid, Sidi Medjahed TIARET Tiaret Tiaret Dahmouni Dahmouni, Ain Bouchekif Meghila Meghila, Sidi Hosni, Sebt KSAR CHELLALA Ksar Chellala Ksar Chellala, Serguine, Zmalet Emir Abdelkader MEDROUSSA Medroussa Medroussa, Melakou, Sidi Bakhti Frenda Frenda, Ain El Hadid, Takhmaret TIARET Ain Kermes Ain Kermes, Medrissa, Djebilet Rosfa, Madna, Sidi Abderrahmane MAHDIA Mahdia Mahdia, Sebaine, Ain Dzarit, Nadorah Hamadia Hamadia, Bougara, Rechaiga RAHOUIA Rahouia Rahouia, Guertoufa Mechraâ Sfa Mechraâ Sfa, Djillali Ben Amar, Tagdemt Oued Lili Oued Lili, Tidda, Sidi Ali Mellal Sougueur Sougueur, Tousnina, Si Abdelghani, Faidja SOUGUEUR Ain Dheb Ain Dheb, Chehaima, Naima. -
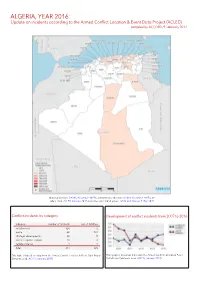
Algeria, Year 2016: Update on Incidents According to the Armed Conflict Location & Event Data Project (ACLED)
ALGERIA, YEAR 2016: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) compiled by ACCORD, 9 February 2017 National borders: GADM, November 2015b; administrative divisions: GADM, November 2015a; in- cident data: ACLED, January 2017; coastlines and inland waters: Smith and Wessel, 1 May 2015 Conflict incidents by category Development of conflict incidents from 2007 to 2016 category number of incidents sum of fatalities riots/protests 324 2 battle 48 122 strategic developments 28 0 violence against civilians 10 4 remote violence 4 1 total 414 129 This table is based on data from the Armed Conflict Location & Event Data Project This graph is based on data from the Armed Conflict Location & Event (datasets used: ACLED, January 2017). Data Project (datasets used: ACLED, January 2017). ALGERIA, YEAR 2016: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 9 FEBRUARY 2017 LOCALIZATION OF CONFLICT INCIDENTS Note: The following list is an overview of the incident data included in the ACLED dataset. More details are available in the actual dataset (date, location data, event type, involved actors, information sources, etc.). In the following list, the names of event locations are taken from ACLED, while the administrative region names are taken from GADM data which serves as the basis for the map above. In Adrar, 11 incidents killing 0 people were reported. The following locations were affected: Adrar, Aougrout, Bordj Badji Mokhtar. In Alger, 34 incidents killing 0 people were reported. The following locations were affected: Ain Benian, Algiers, Bab El Oued, Draria, El Hamiz, Rouiba. -

Décret Exécutif N° 12-428 Du 16 Décembre 2012 Portant Déclaration
9 Safar 1434 7 23 décembre 2012 JOURNAL OFFICIEL DE LA REPUBLIQUE ALGERIENNE N° 70 DECRETS Décret exécutif n° 12-428 du 2 Safar 1434 Vu la loi n° 12-07 du 28 Rabie El Aouel 1433 correspondant au 16 décembre 2012 portant correspondant au 21 février 2012 relative à la wilaya ; déclaration d’utilité publique les opérations de réalisation des projets entrant dans le cadre de la Vu le décret présidentiel n°12-325 du 16 Chaoual 1433 production, du transport et de la distribution de correspondant au 3 septembre 2012 portant nomination du l’électricité et du gaz. Premier ministre ; ———— Vu le décret présidentiel n°12-326 du 17 Chaoual 1433 Le Premier ministre, correspondant au 4 septembre 2012 portant nomination des membres du Gouvernement ; Sur le rapport conjoint du ministre des finances et le ministre de l’énergie et des mines, Vu le décret exécutif n°93-186 du 27 juillet 1993, complété, déterminant les modalités d’application de la loi Vu la Constitution, notamment ses articles 85-3°et 125 n°91-11 du 27 avril 1991, complétée, fixant les règles (alinéa 2) ; relatives à l’expropriation pour cause d’utilité publique ; Vu la loi n°90-30 du 1er décembre 1990, modifiée et Après approbation du Président de la République ; complétée, portant loi domaniale ; Vu la loi n°91-11 du 27 avril 1991, complétée, fixant Décrète : les règles relatives à l’expropriation pour cause d’utilité publique ; Article 1er. – En application des dispositions de l’article 12 bis de la loi n°91-11du 27 avril 1991, et conformément Vu la loi n°98-04 du 20 Safar 1419 correspondant -

Communes of Algeria
S.No. Commune HASC Population 1 Abadla DZ.BC.AB 10,845 2 Abalissa DZ.TM.AB 6,484 3 Abi Youcef DZ.TO.AU 7,743 4 Abou El Hassen DZ.CH.AH 20,164 5 Achaacha DZ.MG.AC 31,360 6 Adekar DZ.BJ.AD 13,495 7 Adrar DZ.AR.AD 43,903 8 Afir DZ.BM.AF 12,613 9 Aflou DZ.LG.AF 53,260 10 Aghbal DZ.TP.AG 6,606 11 Aghbalou DZ.BU.AG 19,530 12 Aghlal DZ.AT.AG 6,841 13 Aghni-Goughrane DZ.TO.AG 10,833 14 Aghrib DZ.TO.AR 13,408 15 Ahl El Ksar DZ.BU.AK 12,315 16 Ahmed Rachedi DZ.ML.AR 14,489 17 Ahmer El Ain DZ.TP.AA 25,633 18 Ahnif DZ.BU.AF 10,268 19 Ain Abessa DZ.SF.AB 15,058 20 Ain Abid DZ.CO.AA 25,958 21 Ain Adden DZ.SB.AA 2,319 22 Ain Arnat DZ.SF.AA 30,129 23 Ain Azel DZ.SF.AZ 41,073 24 Ain Bebouche DZ.OB.AC 14,597 25 Ain Beida DZ.OB.AB 92,197 26 Ain Beida DZ.OG.AB 14,500 27 Ain Beida Harriche DZ.ML.AB 18,513 28 Ain Ben Beida DZ.GL.AB 8,269 29 Ain Benian DZ.AD.AN 4,677 30 Ain Benian DZ.AL.AB 52,343 31 Ain Ben Khelil DZ.NA.AB 3,776 32 Ain-Bessem DZ.BU.AB 36,830 33 Ain Biya DZ.OR.AB 26,253 34 Ain Bouchekif DZ.TR.AB 12,386 35 Ain Boucif DZ.MD.AB 24,434 36 Ain Boudinar DZ.MG.AB 5,241 37 Ain Bouihi DZ.AD.AB 13,920 38 Ain Bouziane DZ.SK.AB 8,381 39 Ain Charchar DZ.SK.AC 13,717 40 Ain Chouhada DZ.DJ.AC 8,337 41 Ain Defla DZ.AD.AD 52,276 42 Ain Deheb DZ.TR.AD 25,366 43 Aïn Djasser DZ.BT.AD 13,232 44 Aïn El Arbaa DZ.AT.AA 12,443 45 Ain El Assel DZ.ET.AA 12,482 www.downloadexcelfiles.com 46 Ain El Berd DZ.SB.AB 13,779 47 Ain El Berda DZ.AN.AB 17,446 48 Ain El Diss DZ.OB.AD 2,741 49 Ain El Fakroun DZ.OB.AF 47,237 50 Ain El Hadid DZ.TR.AH 13,358 51 Ain El Hadjar DZ.BU.AH -

Nom Prenom Date Denaissance Lieu De Naissance KHELIFA ZINEB 08/01/1990 HASSI MESSAOUD ARAB SAID YACINE 15/07/1988 TIARET ABDERRA
REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE MINISTERE DE L'ENSEIGNEMENT SUPERIEUR ET DE LA RECHERCHE SCIENTIFIQUE UNIVERSITE IBN KHALDOUNE TIARET INSTITU DES SCIENCES VETERINAIRE DE TIARET LISTE DES CANDIDATS ACCEPTER POUR LE CONCOURS DE MAGISTER OPTION :Reproduction des animaux de la ferme, N° Nom Prenom Date denaissance Lieu de naissance EMARGEMENT 1 KHELIFA ZINEB 08/01/1990 HASSI MESSAOUD DOC VETE 2 ARAB SAID YACINE 15/07/1988 TIARET 3 ABDERRAHIM ABDELKADER 30/06/1984 TIARET 4 DJEMILI AHMED ZINE ELABIDINE 16/03/1989 ORAN 5 BETTAHAR HEMZA 15/02/1989 OUED RHIOU - RELIZANE 6 AIDET NACERA 07/11/1988 TIARET 7 AICHE SOUAD 08/04/1990 MAHDIA - TIARET 8 MOHAND OUSSAIDSOUMIA 30/11/1989 TIARET 9 ACHAB MALIKA 26/12/1987 AIN TARIK- RELIZANE 10 CHEDDAD KADA 02/06/1990 MASCARA 11 MOKHTAR ANOUAR KADA 31/08/1984 HUSSEIN DEY - ALGER 12 KHACEF MOHAMED MOHSEN 08/08/1990 SETIF 13 BELARBI NOUREDDINE 08/05/1986 TIARET 14 BELALMI RAWIYA 28/07/1990 RAS ELOUAD 15 HARRICHE ZAHIRA 28/09/1984 TIARET 16 ADDA FOUZIA 06/10/1984 TIARET 17 BOUCHENTOUF KHADIDJA 04/05/1984 TIARET 18 TOUAHRI AHMED 29/08/1989 EL ATTAF 19 MAACHOU SOFIANE 21/01/1982 MEDEA 20 LAZREG BAGHDAD 04/05/1987 TIARET 21 LABDELLI BRAHIM 14/07/1987 TIARET 22 MOURIDA MOHAMMED 06/10/1988 ZAOUIET KOUNTA -ADRAR 23 TITAF ABDELDAIM 25/07/1986 BECHAR 24 RAZKI HAMZA 02/01/1990 GHRISS - MASCARA 25 HADJ BOUSSADA YASSICE 09/07/1988 DAHMOUNI 26 BENALDJIA IMENE 27/05/1991 BATNA 27 CHENAFI ILHAM 04/09/1989 CONSTANTINE 28 BENCHERIF HAMZA 12/05/1987 SEBDOU - TLEMCEN 29 TARCHID AHMED YACINE 03/07/1984 TIARET 30