INSM1 Expression and Its Diagnostic Significance in Extraskeletal Myxoid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soft Tissue Cytopathology: a Practical Approach Liron Pantanowitz, MD
4/1/2020 Soft Tissue Cytopathology: A Practical Approach Liron Pantanowitz, MD Department of Pathology University of Pittsburgh Medical Center [email protected] What does the clinician want to know? • Is the lesion of mesenchymal origin or not? • Is it begin or malignant? • If it is malignant: – Is it a small round cell tumor & if so what type? – Is this soft tissue neoplasm of low or high‐grade? Practical diagnostic categories used in soft tissue cytopathology 1 4/1/2020 Practical approach to interpret FNA of soft tissue lesions involves: 1. Predominant cell type present 2. Background pattern recognition Cell Type Stroma • Lipomatous • Myxoid • Spindle cells • Other • Giant cells • Round cells • Epithelioid • Pleomorphic Lipomatous Spindle cell Small round cell Fibrolipoma Leiomyosarcoma Ewing sarcoma Myxoid Epithelioid Pleomorphic Myxoid sarcoma Clear cell sarcoma Pleomorphic sarcoma 2 4/1/2020 CASE #1 • 45yr Man • Thigh mass (fatty) • CNB with TP (DQ stain) DQ Mag 20x ALT –Floret cells 3 4/1/2020 Adipocytic Lesions • Lipoma ‐ most common soft tissue neoplasm • Liposarcoma ‐ most common adult soft tissue sarcoma • Benign features: – Large, univacuolated adipocytes of uniform size – Small, bland nuclei without atypia • Malignant features: – Lipoblasts, pleomorphic giant cells or round cells – Vascular myxoid stroma • Pitfalls: Lipophages & pseudo‐lipoblasts • Fat easily destroyed (oil globules) & lost with preparation Lipoma & Variants . Angiolipoma (prominent vessels) . Myolipoma (smooth muscle) . Angiomyolipoma (vessels + smooth muscle) . Myelolipoma (hematopoietic elements) . Chondroid lipoma (chondromyxoid matrix) . Spindle cell lipoma (CD34+ spindle cells) . Pleomorphic lipoma . Intramuscular lipoma Lipoma 4 4/1/2020 Angiolipoma Myelolipoma Lipoblasts • Typically multivacuolated • Can be monovacuolated • Hyperchromatic nuclei • Irregular (scalloped) nuclei • Nucleoli not typically seen 5 4/1/2020 WD liposarcoma Layfield et al. -

Well-Differentiated Spindle Cell Liposarcoma
Modern Pathology (2010) 23, 729–736 & 2010 USCAP, Inc. All rights reserved 0893-3952/10 $32.00 729 Well-differentiated spindle cell liposarcoma (‘atypical spindle cell lipomatous tumor’) does not belong to the spectrum of atypical lipomatous tumor but has a close relationship to spindle cell lipoma: clinicopathologic, immunohistochemical, and molecular analysis of six cases Thomas Mentzel1, Gabriele Palmedo1 and Cornelius Kuhnen2 1Dermatopathologie, Friedrichshafen, Germany and 2Institute of Pathology, Medical Center, Mu¨nster, Germany Well-differentiated spindle cell liposarcoma represents a rare atypical/low-grade malignant lipogenic neoplasm that has been regarded as a variant of atypical lipomatous tumor. However, well-differentiated spindle cell liposarcoma tends to occur in subcutaneous tissue of the extremities, the trunk, and the head and neck region, contains slightly atypical spindled tumor cells often staining positively for CD34, and lacks an amplification of MDM2 and/or CDK4 in most of the cases analyzed. We studied a series of well-differentiated spindle cell liposarcomas arising in two female and four male patients (age of the patients ranged from 59 to 85 years). The neoplasms arose on the shoulder, the chest wall, the thigh, the lower leg, the back of the hand, and in paratesticular location. The size of the neoplasms ranged from 1.5 to 10 cm (mean: 6.0 cm). All neoplasms were completely excised. The neoplasms were confined to the subcutis in three cases, and in three cases, an infiltration of skeletal muscle was seen. Histologically, the variably cellular neoplasms were composed of atypical lipogenic cells showing variations in size and shape, and spindled tumor cells with slightly enlarged, often hyperchromatic nuclei. -

TERT Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived from Cells with Low Rates of Self-Renewal
TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal Patrick J. Killelaa,1, Zachary J. Reitmana,1, Yuchen Jiaob,1, Chetan Bettegowdab,c,1, Nishant Agrawalb,d, Luis A. Diaz, Jr.b, Allan H. Friedmana, Henry Friedmana, Gary L. Galliac,d, Beppino C. Giovanellae, Arthur P. Grollmanf, Tong-Chuan Heg, Yiping Hea, Ralph H. Hrubanh, George I. Jalloc, Nils Mandahli, Alan K. Meekerh,m, Fredrik Mertensi, George J. Nettoh,l, B. Ahmed Rasheeda, Gregory J. Rigginsc, Thomas A. Rosenquistf, Mark Schiffmanj, Ie-Ming Shihh, Dan Theodorescuk, Michael S. Torbensonh, Victor E. Velculescub, Tian-Li Wangh, Nicolas Wentzensenj, Laura D. Woodh, Ming Zhangb, Roger E. McLendona, Darell D. Bignera, Kenneth W. Kinzlerb, Bert Vogelsteinb,2, Nickolas Papadopoulosb, and Hai Yana,2 aThe Preston Robert Tisch Brain Tumor Center at Duke, Pediatric Brain Tumor Foundation Institute at Duke, and Department of Pathology, Duke University Medical Center, Durham, NC 27710; bLudwig Center for Cancer Genetics and Howard Hughes Medical Institutions, Johns Hopkins Kimmel Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD 21231; Departments of cNeurosurgery, dOtolaryngology—Head and Neck Surgery, hPathology, lUrology, and mOncology, Johns Hopkins University School of Medicine, Baltimore, MD 21231; eChristus Stehlin Foundation for Cancer Research, Houston, TX 77025; fDepartment of Pharmacological Sciences, Stony Brook University, Stony Brook, NY 11794; gMolecular Oncology Laboratory, Department of Orthopaedic -
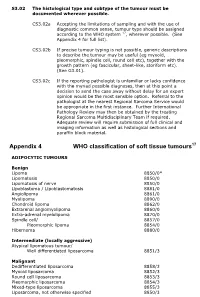
Appendix 4 WHO Classification of Soft Tissue Tumours17
S3.02 The histological type and subtype of the tumour must be documented wherever possible. CS3.02a Accepting the limitations of sampling and with the use of diagnostic common sense, tumour type should be assigned according to the WHO system 17, wherever possible. (See Appendix 4 for full list). CS3.02b If precise tumour typing is not possible, generic descriptions to describe the tumour may be useful (eg myxoid, pleomorphic, spindle cell, round cell etc), together with the growth pattern (eg fascicular, sheet-like, storiform etc). (See G3.01). CS3.02c If the reporting pathologist is unfamiliar or lacks confidence with the myriad possible diagnoses, then at this point a decision to send the case away without delay for an expert opinion would be the most sensible option. Referral to the pathologist at the nearest Regional Sarcoma Service would be appropriate in the first instance. Further International Pathology Review may then be obtained by the treating Regional Sarcoma Multidisciplinary Team if required. Adequate review will require submission of full clinical and imaging information as well as histological sections and paraffin block material. Appendix 4 WHO classification of soft tissue tumours17 ADIPOCYTIC TUMOURS Benign Lipoma 8850/0* Lipomatosis 8850/0 Lipomatosis of nerve 8850/0 Lipoblastoma / Lipoblastomatosis 8881/0 Angiolipoma 8861/0 Myolipoma 8890/0 Chondroid lipoma 8862/0 Extrarenal angiomyolipoma 8860/0 Extra-adrenal myelolipoma 8870/0 Spindle cell/ 8857/0 Pleomorphic lipoma 8854/0 Hibernoma 8880/0 Intermediate (locally -

Spindle Cell Liposarcoma with a TRIO-TERT Fusion Transcript
Virchows Archiv (2019) 475:391–394 https://doi.org/10.1007/s00428-019-02545-5 BRIEF REPORTS Spindle cell liposarcoma with a TRIO-TERT fusion transcript David I. Suster1 & Vikram Deshpande1 & Ivan Chebib1 & Martin S. Taylor1 & John Mullen2 & Miriam A. Bredella3 & G. Petur Nielsen1 Received: 7 January 2019 /Revised: 8 February 2019 /Accepted: 11 February 2019 /Published online: 22 February 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Conventional well-differentiated, dedifferentiated, and myxoid liposarcomas have long been known to harbor numerous typical genetic alterations that allow for diagnosis of these tumors. These include MDM2 and CDK4 amplification in well-differentiated and dedifferentiated liposarcomas as well as FUS-DDIT3 rearrangements in myxoid liposarcoma. More recently, in-frame TRIO- TERT fusion genes have been described in a subset of non-translocation-related sarcomas including myxofibrosarcoma, dedifferentiated liposarcoma, undifferentiated pleomorphic sarcoma, pleomorphic rhabdomyosarcoma, and leiomyosarcoma. These genetic rearrangements lead to TERT mRNA expression levels hundreds of times higher than normal, causing increased telomerase activation in these tumors. Herein, we describe an unusual case of a liposarcoma with spindle cell features and a TRIO-TERT fusion transcript identified through next-generation sequencing. Keywords Liposarcoma . TRIO-TERT . Fusion transcript The current classification of spindle cell lipomatous neoplasms Recently, a novel in-frame TRIO-TERT fusion has been is still evolving. In the literature, the term Bspindle cell identified in some non-translocation-related sarcomas [6]. liposarcoma^ has been used to describe many tumors including TRIO plays an important role in cell adhesion and nuclear spindled variants of well-differentiated liposarcoma and signaling pathways and is part of a family of Rho GTPases myxoid liposarcoma, as well as spindle cell lipomas with that act as guanine nucleotide exchange factors [7]. -

Dedifferentiated Extraskeletal Myxoid Chondrosarcoma of the Masticator Space - a Case Report
The Korean Journal of Pathology 2011; 45(S1): S101-105 DOI: 10.4132/KoreanJPathol.2011.45.S1.S101 Dedifferentiated Extraskeletal Myxoid Chondrosarcoma of the Masticator Space - A Case Report - Geunyoung Jung · Kyung-Ja Cho We describe a 69-year-old woman who presented with a dedifferentiated extraskeletal myxoid Seung-Ho Choi1 · Mi-Jung Kim chondrosarcoma arising in the left masticator space. Computed tomography and magnetic reso- nance imaging revealed a 5 cm sized mass in the left masticator space. Histologically, the tumor Departments of Pathology and consisted of two distinct areas. The less cellular area was a low-grade extraskeletal myxoid chon- 1Otorhinolaryngology, Asan Medical Center, drosarcoma, composed of strands or cords of uniform spindle cells and abundant myxoid stro- University of Ulsan College of Medicine, Seoul, ma. The more cellular, dedifferentiated area corresponded to a high grade myxofibrosarcoma, Korea consisting of anaplastic tumor cells in myxoid stroma and geographic necrosis. The tumor cells of the former area were positive for S-100 protein, microtubule-associated protein-2 (MAP-2) and Received: October 25, 2010 class III β-tubulin, but negative for cytokeratin, smooth muscle actin, and desmin. The tumor cells November 23, 2010 Accepted: in the latter, pleomorphic area showed MAP-2 and β-tubulin immunoreactivity with a high Ki-67 Corresponding Author labeling index. Based on its histologic and immunohistochemical features, the tumor was consid- Mi-Jung Kim, M.D. ered a dedifferentiated extraskeletal myxoid chondrosarcoma. Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 388-1 Pungnap 2-dong, Songpa-gu, Seoul 138-736, Korea Tel: +82-2-3010-4557 Fax: +82-2-472-7898 E-mail: [email protected] Key Words: Extraskeletal myxoid chondrosarcoma; Cell dedifferentiation; Masticator space Extraskeletal myxoid chondrosarcoma (EMC) is a rare malig- CASE REPORT nant soft tissue sarcoma, distinguished from its skeletal coun- terpart by cytogenetic and histologic features. -

Soft Tissue and Bone Tumors
Soft tissue and bone tumors Zoltán Sápi MD, PhD 1st Department of Pathology and Experimental Cancer Research • Large and heterogeneous group with more than 200 entity and more than 50 sarcoma types • Previously: classified according to a histogenetic concept (fibrosarcoma from fibroblast) • Now: primitive multipotential stem cells differentiate along one or more lines Pluripotent mesenchymal stem cell, may differentiate into different direction lipogenic, neurogenic, fibro-myofibroblastic, smooth muscle, vascular, uncertain • Light microscopic evaluation (H&E) + many ancillary techniques Principles Characteristic morphology with corresponding biological behavior Variants (with the same biological behavior) Similar morphology but different biological behavior (MFS – LGFMS) Different morphology but the same genetic background (Giant cell fibroblastoma – DFSP) Genetic promiscuity (partly or totally) Molecular complexity complex karyotype Recurrent cytogenetic alteration, genetic instability Translocations fusion genes, proteins no fusion gene de novo formation de novo formation, rarely fusion gene is the initial step/driving force „dysplastic-precursors” synovial sarcoma, myxoid liposarcoma MPNST, leiomyosarcoma FISH probes Break-apart SS18 (SYT) Synovial sarcoma FOXO1 Alveolar rhabdomyosarcoma DDIT3 (CHOP) Myxoid liposarcoma FUS Myxoid liposarcoma, Angiomatoid fibrosus histiocytoma, LGFMS EWSR1 Ewing sc, AFH, DSRCT, EMC, MC, Clear cell sc EWSR1 COL1A1 Dermatofibrosarcoma protuberans USP6 Nodular fasciitis, Aneurysmal bone cyst ETV6 Infantile -

The Role of Cytogenetics and Molecular Diagnostics in the Diagnosis of Soft-Tissue Tumors Julia a Bridge
Modern Pathology (2014) 27, S80–S97 S80 & 2014 USCAP, Inc All rights reserved 0893-3952/14 $32.00 The role of cytogenetics and molecular diagnostics in the diagnosis of soft-tissue tumors Julia A Bridge Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA Soft-tissue sarcomas are rare, comprising o1% of all cancer diagnoses. Yet the diversity of histological subtypes is impressive with 4100 benign and malignant soft-tissue tumor entities defined. Not infrequently, these neoplasms exhibit overlapping clinicopathologic features posing significant challenges in rendering a definitive diagnosis and optimal therapy. Advances in cytogenetic and molecular science have led to the discovery of genetic events in soft- tissue tumors that have not only enriched our understanding of the underlying biology of these neoplasms but have also proven to be powerful diagnostic adjuncts and/or indicators of molecular targeted therapy. In particular, many soft-tissue tumors are characterized by recurrent chromosomal rearrangements that produce specific gene fusions. For pathologists, identification of these fusions as well as other characteristic mutational alterations aids in precise subclassification. This review will address known recurrent or tumor-specific genetic events in soft-tissue tumors and discuss the molecular approaches commonly used in clinical practice to identify them. Emphasis is placed on the role of molecular pathology in the management of soft-tissue tumors. Familiarity with these genetic events -

Pathology and Genetics of Tumours of Soft Tissue and Bone
bb5_1.qxd 13.9.2006 14:05 Page 3 World Health Organization Classification of Tumours WHO OMS International Agency for Research on Cancer (IARC) Pathology and Genetics of Tumours of Soft Tissue and Bone Edited by Christopher D.M. Fletcher K. Krishnan Unni Fredrik Mertens IARCPress Lyon, 2002 bb5_1.qxd 13.9.2006 14:05 Page 4 World Health Organization Classification of Tumours Series Editors Paul Kleihues, M.D. Leslie H. Sobin, M.D. Pathology and Genetics of Tumours of Soft Tissue and Bone Editors Christopher D.M. Fletcher, M.D. K. Krishnan Unni, M.D. Fredrik Mertens, M.D. Coordinating Editor Wojciech Biernat, M.D. Layout Lauren A. Hunter Illustrations Lauren A. Hunter Georges Mollon Printed by LIPS 69009 Lyon, France Publisher IARCPress International Agency for Research on Cancer (IARC) 69008 Lyon, France bb5_1.qxd 13.9.2006 14:05 Page 5 This volume was produced in collaboration with the International Academy of Pathology (IAP) The WHO Classification of Tumours of Soft Tissue and Bone presented in this book reflects the views of a Working Group that convened for an Editorial and Consensus Conference in Lyon, France, April 24-28, 2002. Members of the Working Group are indicated in the List of Contributors on page 369. bb5_1.qxd 22.9.2006 9:03 Page 6 Published by IARC Press, International Agency for Research on Cancer, 150 cours Albert Thomas, F-69008 Lyon, France © International Agency for Research on Cancer, 2002, reprinted 2006 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. -

Aggressive Angiomyxoma in Pregnancy: a Rare and Commonly Misdiagnosed Entity Kamal Malukani, Amit V
Published online: 2020-02-19 Case Report Access this article online Quick Response Code: Aggressive angiomyxoma in pregnancy: A rare and commonly misdiagnosed entity Kamal Malukani, Amit V. Varma, Devashish Choudhary, Shilpi Dosi Website: www.jlponline.org Abstract: DOI: 10.4103/JLP.JLP_179_17 Aggressive angiomyxoma (AAM) is an uncommon mesenchymal tumor that predominantly involves the pelvis and perineum of young females. It is often clinically mistaken for more common superficial lesions such as vaginal cysts, labial cysts, and lipomas. A review of the medical literature reveals very few cases of AAM reported in pregnancy. We describe a rare case of AAM in pregnancy, clinically misdiagnosed as prolapsed cervical fibroid. Key words: Aggressive angiomyxoma, mesenchymal tumors, pregnancy Introduction (G2 P1 L1), married for 5 years, presented with bleeding per vagina for 3 days. She ggressive angiomyxoma (AAM) is also complained of huge mass coming out Aa rare mesenchymal neoplasm of of vagina, difficulty in walking, and pain vulvo‑perineal region and usually found in abdomen and lower back. There was [1] in women of reproductive age group. It no history of fever, weight loss, trauma, is a slow‑growing, low‑grade neoplasm bowel or bladder disturbance, and use of but is locally infiltrative, and relapse any contraceptive. Her menstrual history has been reported in about 30%–40% of and past history were unremarkable. Local cases even after many years of complete examination showed a large pedunculated, resection.[2] Rarely, AAM can metastasize well‑circumscribed, ulcerated mass coming to lungs, peritoneum, and lymph nodes.[3] out of the vagina. Only a few cases of AAM coexistent Ultrasound of abdomen showed with pregnancy are reported in medical bulky anteverted uterus, measuring literature.[4,5] AAM grows to a huge size 9.6 cm × 7.6 cm × 5.4 cm. -

Desterrando El Término Ewing-Like Sarcoma: ¿Qué Entidades Se Cobijaban Bajo Esa Denominación?
Desterrando el término Ewing-like sarcoma: ¿Qué entidades se cobijaban bajo esa denominación? Sílvia Bagué Servei de Patologia Hospital de la Santa Creu i Sant Pau Universitat Autònoma de Barcelona #SEOM20 No disclosures Small round cell sarcomas (SRCS) - Group of malignant neoplasms sharing histological similar features - Mainly in children & young adults - High-grade by definition. Often “translocated-sarcomas” • Ewing sarcoma • “Ewing-like” sarcomas • Desmoplastic SRCT • Alveolar rhabdomyosarcoma • Poorly diff sinovial sarcoma • Mesenchymal chondrosarcoma • High-grade myxoid liposarcoma • Small cell osteosarcoma • Undiff sarcoma with round cell morphology (USRCS) #SEOM203 Ewing sarcoma • Most common round cell sarcoma • 1st - 2nd decade • 85% diaphysis-metaphysis long bones > pelvis, ribs, chest wall (‘Askin’) • 15% extraeskeletal (adults) • Key morphologic features: uniform monotonous round cells, fine chromatin, inconspicious nucleoli, scant clear to pale cytoplasm • Key immunohistochemical stains: strong and diffuse membranous CD99 expression; NKX2+, PAX7+ • Genetics: fusion between EFT genes (FUS, EWSR1) and TAF15 genes and ETS transcription factor family members (Fli-1, ERG, ETV1, ETV4, FEV, E1AF, ZSG) 85-90% t(11;22)(q24;q12) EWSR1- FLI1 fusion gene 5-10 % t(21;22)(q22;q12) EWSR1-ERG fusion gene CD99 FISH EWSR1 #SEOM204 Desmoplastic small round cell tumor Clinical history Boy, 12 y-o. Intraabdominal mass #SEOM205 DSRCT: IHC & genetics Malignant round cell tumor with desmoplastic stroma, poliphenotypic differentiation and translocation -

The 2020 WHO Classification of Soft Tissue Tumours: News and Perspectives
PATHOLOGICA 2021;113:70-84; DOI: 10.32074/1591-951X-213 Review The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives Marta Sbaraglia1, Elena Bellan1, Angelo P. Dei Tos1,2 1 Department of Pathology, Azienda Ospedale Università Padova, Padova, Italy; 2 Department of Medicine, University of Padua School of Medicine, Padua, Italy Summary Mesenchymal tumours represent one of the most challenging field of diagnostic pathol- ogy and refinement of classification schemes plays a key role in improving the quality of pathologic diagnosis and, as a consequence, of therapeutic options. The recent publica- tion of the new WHO classification of Soft Tissue Tumours and Bone represents a major step toward improved standardization of diagnosis. Importantly, the 2020 WHO classi- fication has been opened to expert clinicians that have further contributed to underline the key value of pathologic diagnosis as a rationale for proper treatment. Several rel- evant advances have been introduced. In the attempt to improve the prediction of clinical behaviour of solitary fibrous tumour, a risk assessment scheme has been implemented. NTRK-rearranged soft tissue tumours are now listed as an “emerging entity” also in con- sideration of the recent therapeutic developments in terms of NTRK inhibition. This deci- sion has been source of a passionate debate regarding the definition of “tumour entity” as well as the consequences of a “pathology agnostic” approach to precision oncology. In consideration of their distinct clinicopathologic features, undifferentiated round cell sarcomas are now kept separate from Ewing sarcoma and subclassified, according to the underlying gene rearrangements, into three main subgroups (CIC, BCLR and not Received: October 14, 2020 ETS fused sarcomas) Importantly, In order to avoid potential confusion, tumour entities Accepted: October 19, 2020 such as gastrointestinal stroma tumours are addressed homogenously across the dif- Published online: November 3, 2020 ferent WHO fascicles.