Phosphatidylalcohols by Hela Cells and A431 Cells Through Activation of Phospholipase D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elimination of GPI2 Suppresses Glycosylphosphatidylinositol Glcnac
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.18.436003; this version posted March 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Elimination of GPI2 suppresses glycosylphosphatidylinositol GlcNAc transferase activity and alters GPI glycan modification in Trypanosoma brucei Aurelio Jenni‡§, SeBastian Knüsel¶, Rupa Nagar||, Mattias Benninger¶, RoBert Häner**, Michael A. J. Ferguson||, IsaBel Roditi¶, Anant K. Menon#, Peter Bütikofer‡1 From the ‡Institute of Biochemistry and Molecular Medicine, University of Bern, 3012 Bern, Switzerland, the §Graduate School for Chemical and Biomedical Sciences, University of Bern, 3012 Bern, Switzerland, the ¶Institute of Cell Biology, University of Bern, 3012 Bern, Switzerland, the ||Wellcome Centre for Anti-Infectives Research, School of Life Sciences, University of Dundee, Dow Street, Dundee, United Kingdom, the **Department for Chemistry and Biochemistry, University of Bern, 3012 Bern, Switzerland, the #Department of Biochemistry, Weill Cornell Medical College, New York, New York 10065, USA. Running Title: GPI synthesis in the absence of GPI2 * The work was supported By Swiss National Science Foundation Sinergia grant CRSII5_170923 to RH, AKM and PB, by a Wellcome Tust Investigator Award (101842/Z13/Z) to MAJF and Swiss National Science Foundation grant 310030_184669 to IR. The authors declare that they have no conflicts of interest with the contents of this article. 1 To whom correspondence should Be addressed: Institute of Biochemistry and Molecular Medicine, Bühlstrasse 28, 3012 Bern, Switzerland. Tel.: 41-31-631-4113; E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.18.436003; this version posted March 19, 2021. -

Suramin Alters Phosphoinositide Synthesis and Inhibits Growth Factor Receptor Binding in HT-29 Cells'
(CANCER RESEARCH 50. 6490-6496. October 15. 1990] Suramin Alters Phosphoinositide Synthesis and Inhibits Growth Factor Receptor Binding in HT-29 Cells' Reinhard Kopp2 and Andreas Pfeiffer Departments of Surgery and Internal Medicine II, Klinikum (irosshadern. University of Munich, West Germany ABSTRACT levels and a stimulation of calcium/calmodulin kinases. Di acylglycerol activates protein kinase C, a family of Ca2+-sensi- Initiation of cell growth frequently involves activation of growth factor tive and phospholipid-dependent isoenzymes, known to phos- receptor-coupled u rosine kinases and stimulation of the phosphoinositide phorylate regulatory proteins and to elevate cytosolic pH levels second messenger system. The antitrypanosomal and antifiliarial drug suramin has been shown to exert antiproliferative activities by inhibition (5, 6). Activation of protein kinase C by phorbol esters and of growth factor receptor binding. We therefore investigated the effect of elevation of intracellular calcium levels by calcium ionophores suramin on epidermal growth factor receptor-binding characteristics and, have been shown to be mitogenically active cofactors during the additionally, searched for effects on basal or cholinergically stimulated initiation of DNA synthesis (7-10). phospholipid metabolism in HT-29 cells. HT-29 colon carcinoma cells have recently been shown to Suramin caused a dose-dependent and noncompetitive inhibition of produce EGF/transforming growth factor «and insulin-like '"I-epidermal growth factor binding (concentration producing 50% inhi growth factor 1-like activities (11), indicating a possible auto bition, 44.2 Mg/ml)but did not alter muscarinic receptor binding. Suramin did not affect the basal '-'I* incorporation into phosphoinositides at crine proliferative effect of these growth factors. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

(4,5) Bisphosphate-Phospholipase C Resynthesis Cycle: Pitps Bridge the ER-PM GAP
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Topological organisation of the phosphatidylinositol (4,5) bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM GAP Shamshad Cockcroft and Padinjat Raghu* Dept. of Neuroscience, Physiology and Pharmacology, Division of Biosciences, University College London, London WC1E 6JJ, UK; *National Centre for Biological Sciences, TIFR-GKVK Campus, Bellary Road, Bangalore 560065, India Address correspondence to: Shamshad Cockcroft, University College London UK; Phone: 0044-20-7679-6259; Email: [email protected] Abstract Phospholipase C (PLC) is a receptor-regulated enzyme that hydrolyses phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) at the plasma membrane (PM) triggering three biochemical consequences, the generation of soluble inositol 1,4,5-trisphosphate (IP3), membrane– associated diacylglycerol (DG) and the consumption of plasma membrane PI(4,5)P2. Each of these three signals triggers multiple molecular processes impacting key cellular properties. The activation of PLC also triggers a sequence of biochemical reactions, collectively referred to as the PI(4,5)P2 cycle that culminates in the resynthesis of this lipid. The biochemical intermediates of this cycle and the enzymes that mediate these reactions are topologically distributed across two membrane compartments, the PM and the endoplasmic reticulum (ER). At the plasma membrane, the DG formed during PLC activation is rapidly converted to phosphatidic acid (PA) that needs to be transported to the ER where the machinery for its conversion into PI is localised. Conversely, PI from the ER needs to be rapidly transferred to the plasma membrane where it can be phosphorylated by lipid kinases to regenerate PI(4,5)P2. -

Synthesis of Lysophospholipids
Molecules 2010, 15, 1354-1377; doi:10.3390/molecules15031354 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Synthesis of Lysophospholipids Paola D’Arrigo 1,2,* and Stefano Servi 1,2 1 Dipartimento di Chimica, Materiali ed Ingegneria Chimica “Giulio Natta”, Politecnico di Milano, Via Mancinelli 7, 20131 Milano, Italy 2 Centro Interuniversitario di Ricerca in Biotecnologie Proteiche "The Protein Factory", Politecnico di Milano and Università degli Studi dell'Insubria, Via Mancinelli 7, 20131 Milano, Italy * Author to whom correspondence should be addressed; E-Mail: paola.d’[email protected]. Received: 17 February 2010; in revised form: 4 March 2010 / Accepted: 5 March 2010 / Published: 8 March 2010 Abstract: New synthetic methods for the preparation of biologically active phospholipids and lysophospholipids (LPLs) are very important in solving problems of membrane–chemistry and biochemistry. Traditionally considered just as second-messenger molecules regulating intracellular signalling pathways, LPLs have recently shown to be involved in many physiological and pathological processes such as inflammation, reproduction, angiogenesis, tumorogenesis, atherosclerosis and nervous system regulation. Elucidation of the mechanistic details involved in the enzymological, cell-biological and membrane-biophysical roles of LPLs relies obviously on the availability of structurally diverse compounds. A variety of chemical and enzymatic routes have been reported in the literature for the synthesis of LPLs: the enzymatic transformation of natural glycerophospholipids (GPLs) using regiospecific enzymes such as phospholipases A1 (PLA1), A2 (PLA2) phospholipase D (PLD) and different lipases, the coupling of enzymatic processes with chemical transformations, the complete chemical synthesis of LPLs starting from glycerol or derivatives. In this review, chemo- enzymatic procedures leading to 1- and 2-LPLs will be described. -

Activation by Inhibition of Second Messenger Compounds
1230 Annals ofthe Rheumatic Diseases 1992; 51: 1230-1236 Sulphasalazine inhibition of human granulocyte Ann Rheum Dis: first published as 10.1136/ard.51.11.1230 on 1 November 1992. Downloaded from activation by inhibition of second messenger compounds Gunnar Carlin, Richard Djursater, Goran Smedegard Abstract We have previously found by using the The effects of sulphasalazine on the pro- granulocyte as a model system that sulpha- duction of second messenger compounds in salazine inhibits cellular activation before activa- human granulocytes have been characterised tion of protein kinase C by interference with the by various stimuli. The increases in cytosolic synthesis of phosphoinositide derived second calcium, inositol trisphosphate, diacylgly- messenger compounds.6 As several cell types cerol, and phosphatidic acid (all important utilise phosphoinositide dependent signal trans- mediators of intracellular signal transduction) duction, sulphasalazine may modify a principal triggered by stimulation were inhibited by common mechanism for the regulation of sulphasalazine. The metabolites 5-amino- cell activity, which may explain the beneficial salicylic acid and sulphapyridine were less effects of the drug in a variety of diseases. potent inhibitors than the mother compound. Many of the stimuli used in the earlier studies It is concluded that sulphasalazine inhibits trigger an increase in cytosolic calcium as part of the synthesis of phosphoinositide derived the activation sequence. The increase in calcium second messenger compounds at the level of has, in a variety of cell systems, been found to phospholipase C or its regulatory guanosine depend on remodelling of phosphoinositides'3 5'-triphosphate (GTP) binding protein. In- (see fig 1). Ligation of a cell receptor leads to hibition of phosphatidic acid synthesis was the phospholipase C dependent hydrolysis of either due to the same mechanism, or to phosphatidylinositol-4,5-bisphosphate genera- interaction with a phospholipase D regulating ting two triggers of cell responses, namely GTP binding protein. -
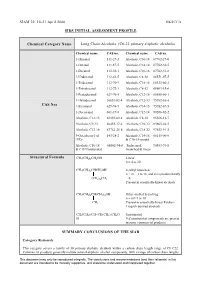
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

Identification of a Phosphatidic Acid-Preferring Phospholipase Al
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 9574-9578, September 1994 Biochemistry Identification of a phosphatidic acid-preferring phospholipase Al from bovine brain and testis (lysophosphatidic acld/lysophosphoipase/Triton X-100 miceles/phospholpase D/diacylgycerol kinase) HENRY N. HIGGS AND JOHN A. GLOMSET* Departments of Biochemistry and Medicine and Regional Primate Research Center, Howard Hughes Medical Institute, University of Washington, SL-15, Seattle, WA 98195 Contributed by John A. Glomset, May 31, 1994 ABSTRACT Recent experiments in several laboratories drolysis by phospholipase A (PLA) activities, producing have provided evidence that phosphatidic acid functions in cell lysophosphatidic acid (LPA). A PA-specific PLA2 has been signaling. However, the mechaninsm that regulate cellular reported (16), but detailed information about this enzyme is phosphatidic acid levels remain obscure. Here we describe a lacking. Another possibility is that a PLA1 might metabolize soluble phospholipase Al from bovine testis that preferentially PA to sn-2-LPA. Several PLA1 activities have been identified hydrolyzes phosphatidic acid when assayed in Triton X-100 in mammalian tissues, including one found in rat liver plasma micelles. Moreover, the enzyme hydrolyzes phosphatidic acid membrane (17), another found in rat brain cytosol (18, 19), molecular species containing two unsaturated fatty acids in and others found in lysosomes (20, 21). None of these preference to those containing a combination of saturated and enzymes, however, displays a preference for PA as a sub- unsaturated fatty acyl groups. Under certain conditions, the strate. enzyme also displays lysophospholipase activity toward In the present study, we identify and characterize a cyto- lysophosphatidic acid. The phospholipase Al is not likely to be solic PLA1 with a strong preference for PA. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Dissociation of Inositol Trisphosphate from Diacylglycerol Production in Rous Sarcoma Virus-Transformed Fibroblasts Timothy J
Dissociation of Inositol Trisphosphate from Diacylglycerol Production in Rous Sarcoma Virus-transformed Fibroblasts Timothy J. Martins, Yoshikazu Sugimoto, and R. L. Erikson Department of Cellular and Developmental Biology, Harvard University, Cambridge, Massachusetts 02138 Abstract. The metabolism of phosphatidylinositol (PI) changes intermediate between those of transformed and and related intermediates was studied in uninfected nontransformed cells. NY72-4 CEF exhibited no in- and Rous sarcoma virus-(RSV) infected chicken em- crease in phosphoinositol concentrations before 8 h in- bryo fibroblasts (CEFs). Cells infected with wild-type cubation at 35°C, indicating that the transformation- Downloaded from http://rupress.org/jcb/article-pdf/108/2/683/1057290/683.pdf by guest on 26 September 2021 RSV exhibited twofold increases in steady-state con- specific changes in inositol metabolism were a delayed centrations of inositol trisphosphate (IP3) and inositol event. Furthermore, inositol turnover was not activated bisphosphate (IP2) as compared to uninfected CEFs. In during this time. In contrast to the case of inositol me- addition, increased concentrations of IP3 and IP2 were tabolism, significant increases in diacylglycerol (DAG) observed in CEFs infected with the RSV temperature- concentrations were observed within 15-30 min after sensitive transformation mutant NY72-4 when main- shift of NY72-4 CEFs to 35°C. tained at the permissive temperature (35°C) for >24 h. These findings suggest that (a) the major changes in Slight increases were -

Regulation of Signaling and Metabolism by Lipin-Mediated Phosphatidic Acid Phosphohydrolase Activity
biomolecules Review Regulation of Signaling and Metabolism by Lipin-mediated Phosphatidic Acid Phosphohydrolase Activity Andrew J. Lutkewitte and Brian N. Finck * Center for Human Nutrition, Division of Geriatrics and Nutritional Sciences, Department of Medicine, Washington University School of Medicine, Euclid Avenue, Campus Box 8031, St. Louis, MO 63110, USA; [email protected] * Correspondence: bfi[email protected]; Tel: +1-3143628963 Received: 4 September 2020; Accepted: 24 September 2020; Published: 29 September 2020 Abstract: Phosphatidic acid (PA) is a glycerophospholipid intermediate in the triglyceride synthesis pathway that has incredibly important structural functions as a component of cell membranes and dynamic effects on intracellular and intercellular signaling pathways. Although there are many pathways to synthesize and degrade PA, a family of PA phosphohydrolases (lipin family proteins) that generate diacylglycerol constitute the primary pathway for PA incorporation into triglycerides. Previously, it was believed that the pool of PA used to synthesize triglyceride was distinct, compartmentalized, and did not widely intersect with signaling pathways. However, we now know that modulating the activity of lipin 1 has profound effects on signaling in a variety of cell types. Indeed, in most tissues except adipose tissue, lipin-mediated PA phosphohydrolase activity is far from limiting for normal rates of triglyceride synthesis, but rather impacts critical signaling cascades that control cellular homeostasis. In this review, we will discuss how lipin-mediated control of PA concentrations regulates metabolism and signaling in mammalian organisms. Keywords: phosphatidic acid; diacylglycerol; lipin; signaling 1. Introduction Foundational work many decades ago by the laboratory of Dr. Eugene Kennedy defined the four sequential enzymatic steps by which three fatty acyl groups were esterified onto the glycerol-3-phosphate backbone to synthesize triglyceride [1]. -

(GPI)-Anchored Glycoprotein ACA ISOLATION, PURIFICATION, PRIMARY STRUCTURE DETERMINATION, and MOLECULAR PARAMETERS of ITS LIPID STRUCTURE*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 43, Issue of October 25, pp. 40472–40478, 2002 © 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. A Novel Human Erythrocyte Glycosylphosphatidylinositol (GPI)-anchored Glycoprotein ACA ISOLATION, PURIFICATION, PRIMARY STRUCTURE DETERMINATION, AND MOLECULAR PARAMETERS OF ITS LIPID STRUCTURE* Received for publication, March 12, 2002, and in revised form, August 2, 2002 Published, JBC Papers in Press, August 7, 2002, DOI 10.1074/jbc.M202416200 Zorica A. Becker Kojic´‡ and Peter Terness From the Institute of Immunology, Department of Transplantation Immunology, University of Heidelberg, Im Neuenheimer Feld 305, 69120 Heidelberg, Germany A method has been elaborated to isolate and purify up no cytoplasmic tail, and are therefore located exclusively on the to homogeneity a novel membrane glycoprotein contain- extracellular side of the plasma membrane. Glycosylated phos- ing a glycosyl-phosphatidylinositol (GPI) anchor by phoinositol-anchored molecules are a structurally diverse fam- means of salting out with ammonium sulfate (40–80% ily of biomolecules (1–3) that includes: protozoan coat compo- saturation), followed by preparative SDS-PAGE, chro- nents, activation antigens, complement regulatory proteins, matography and acetone precipitation. The preparation adhesion molecules, membrane-associated enzymes, and many obtained was homogeneous upon electrophoresis in the others glycoproteins. This type of cell membrane attachment Downloaded from presence of 0.1% SDS after reduction with 2-mercapto- implies that the protein moieties of these antigens are located ethanol. It is protein-soluble at its isoelectrical point outside the membrane, have potentially high lateral mobility, (pH 5.5) with molecular mass of 65,000 daltons.