Acetate Kinase Activity and Kinetic Properties of the Enzyme in Desulfovibrio Piger Vib-7 and Desulfomicrobium Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Katalog 2015 Cover Paul Lin *Hinweis Förderung.Indd
Product List 2015 WE LIVE SERVICE Certificates quartett owns two productions sites that are certified according to EN ISO 9001:2008 Quality management systems - Requirements EN ISO 13485:2012 + AC:2012 Medical devices - Quality management systems - Requirements for regulatory purposes GMP Conformity Our quality management guarantees products of highest quality! 2 Foreword to the quartett product list 2015 quartett Immunodiagnostika, Biotechnologie + Kosmetik Vertriebs GmbH welcomes you as one of our new business partners as well as all of our previous loyal clients. You are now member of quartett´s worldwide customers. First of all we would like to introduce ourselves to you. Founded as a family-run company in 1986, quartett ensures for more than a quarter of a century consistent quality of products. Service and support of our valued customers are our daily businesses. And we will continue! In the end 80´s quartett offered radioimmunoassay and enzyme immunoassay kits from different manufacturers in the USA. In the beginning 90´s the company changed its strategy from offering products for routine diagnostic to the increasing field of research and development. Setting up a production plant in 1997 and a second one in 2011 supported this decision. The company specialized its product profile in the field of manufacturing synthetic peptides for antibody production, peptides such as protease inhibitors, biochemical reagents and products for histology, cytology and immunohistology. All products are exclusively manufactured in Germany without outsourcing any production step. Nowadays, we expand into all other diagnostic and research fields and supply our customers in universities, government institutes, pharmaceutical and biotechnological companies, hospitals, and private doctor offices. -

Recombinant Escheichia Coli-Catalyzed Production of Cytidine 5′-Triphosphate from Cytidine 5′-Monophosphate
J. Ind. Eng. Chem., Vol. 12, No. 5, (2006) 757-761 Recombinant Escheichia coli-Catalyzed Production of Cytidine 5′-Triphosphate from Cytidine 5′-Monophosphate Sun-Gu Lee† and Byung-Gee Kim* Department of Chemical and Biochemical Engineering, Pusan National University, Busan 609-735, Korea *School of Chemical Engineering, and Institute of Molecular Biology and Genetics, Seoul National University, Seoul 151-742, Korea Received April 24, 2006; Accepted May 22, 2006 Abstract: A recombinant Escherichia coli overexpressing CMP-kinase was constructed and employed as a whole cell biocatalyst for the conversion of cytidine 5′-monophosphate to cytidine 5′-triphosphate. In the whole cell biocatalysis, recombinant CMP kinase catalyzed the conversion of CMP to CDP, and endogenous acetate kinase of the E. coli was utilized for the ATP regeneration as well as for the conversion of CDP to CTP. A conversion yield of ca. 88 % CTP was obtained when starting with 20 mM CMP, 1 mM ATP, and 80 mM acetyl phosphate based on the initial CMP concentration. Endogenous pyruvate kinase and poly- phosphate kinase were inefficient in the process. The CTP production system was applied to the production of CMP-NeuAc by additionally introducing the CMP-NeuAc synthetase gene into the recombinant E. coli. Keywords: CMP-kinase, whole cell biocatalysis, ATP regeneration, cytidine 5′-monophosphate Introduction employing enzymes [4]. They applied various enzymatic 1) methods and chemical methods and concluded that the As glycosyltransferase-catalyzed synthetic techniques enzymatic method based on adenylate kinase/pyruvate are becoming recognized as powerful methods for the kinase provided the most convenient route to CTP. In the preparation of biologically important oligosaccharides, process, CTP was generated efficiently from an inexpen- the development of cost-efficient production methods for sive substrate, CMP. -

Découverte D'une Nouvelle Famille De Protéine Kinases Bactériennes
Découverte d’une nouvelle famille de protéine kinases bactériennes : mécanismes de fonctionnement et rôle cellulaire de YdiB, un archétype chez Baccillus subtilis Hien-Anh Nguyen To cite this version: Hien-Anh Nguyen. Découverte d’une nouvelle famille de protéine kinases bactériennes : mécanismes de fonctionnement et rôle cellulaire de YdiB, un archétype chez Baccillus subtilis. Sciences agricoles. Université de Grenoble, 2012. Français. NNT : 2012GRENV017. tel-00721757 HAL Id: tel-00721757 https://tel.archives-ouvertes.fr/tel-00721757 Submitted on 30 Jul 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITÉ DE GRENOBLE Spécialité : Chimie-Biologie Arrêté ministériel : 7 août 2006 Présentée par Hien-Anh NGUYEN Thèse dirigée par le Dr. Jean-Michel JAULT préparée au sein de l’Institut de Biologie Structurale J.-P. Ebel, et du CEA de Grenoble dans l'École Doctorale Chimie et Sciences du Vivant Découverte d’une nouvelle famille de protéines kinases bactériennes : Mécanisme de fonctionnement et rôle cellulaire de YdiB, un représentant chez B. subtilis Thèse soutenue publiquement le 23 mai 2012 devant le jury composé de : Mme. Patricia DOUBLET Rapporteur Prof. -

Two-Dimensional Isobutyl Acetate Production Pathways to Improve Carbon Yield
ARTICLE Received 23 Apr 2015 | Accepted 13 May 2015 | Published 25 Jun 2015 DOI: 10.1038/ncomms8488 OPEN Two-dimensional isobutyl acetate production pathways to improve carbon yield Yohei Tashiro1, Shuchi H. Desai1,2 & Shota Atsumi1,2 For an economically competitive biological process, achieving high carbon yield of a target chemical is crucial. In biochemical production, pyruvate and acetyl-CoA are primary building blocks. When sugar is used as the sole biosynthetic substrate, acetyl-CoA is commonly generated by pyruvate decarboxylation. However, pyruvate decarboxylation during acetyl-CoA formation limits the theoretical maximum carbon yield (TMCY) by releasing carbon, and in some cases also leads to redox imbalance. To avoid these problems, we describe here the construction of a metabolic pathway that simultaneously utilizes glucose and acetate. Acetate is utilized to produce acetyl-CoA without carbon loss or redox imbalance. We demonstrate the utility of this approach for isobutyl acetate (IBA) production, wherein IBA production with glucose and acetate achieves a higher carbon yield than with either sole carbon source. These results highlight the potential for this multiple carbon source approach to improve the TMCY and balance redox in biosynthetic pathways. 1 Department of Chemistry, University of California, Davis, One Shields Avenue, Davis, California 95616, USA. 2 Microbiology Graduate Group, University of California, Davis, One Shields Avenue, Davis, California 95616, USA. Correspondence and requests for materials should be addressed to S.A. (email: [email protected]). NATURE COMMUNICATIONS | 6:7488 | DOI: 10.1038/ncomms8488 | www.nature.com/naturecommunications 1 & 2015 Macmillan Publishers Limited. All rights reserved. ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms8488 iochemical production from biomass, a renewable resource OH synthesized from CO2 and sunlight, may contribute to a 1 Bcarbon-neutral society . -

Acetate Activation in Methanosaeta Thermophila: Characterization of the Key Enzymes Pyrophosphatase and Acetyl-Coa Synthetase
Hindawi Publishing Corporation Archaea Volume 2012, Article ID 315153, 10 pages doi:10.1155/2012/315153 Research Article Acetate Activation in Methanosaeta thermophila: Characterization of the Key Enzymes Pyrophosphatase and Acetyl-CoA Synthetase Stefanie Berger, Cornelia Welte, and Uwe Deppenmeier Institute for Microbiology and Biotechnology, University of Bonn, Meckenheimer Allee 168, 53115 Bonn, Germany Correspondence should be addressed to Uwe Deppenmeier, [email protected] Received 16 May 2012; Accepted 30 June 2012 Academic Editor: Francesca Paradisi Copyright © 2012 Stefanie Berger et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The thermophilic methanogen Methanosaeta thermophila uses acetate as sole substrate for methanogenesis. It was proposed that the acetate activation reaction that is needed to feed acetate into the methanogenic pathway requires the hydrolysis of two ATP, whereas the acetate activation reaction in Methanosarcina sp. is known to require only one ATP. As these organisms live at the thermodynamic limit that sustains life, the acetate activation reaction in Mt. thermophila seems too costly and was thus reevaluated. It was found that of the putative acetate activation enzymes one gene encoding an AMP-forming acetyl-CoA synthetase was highly expressed. The corresponding enzyme was purified and characterized in detail. It catalyzed the ATP-dependent formation of acetyl- CoA, AMP, and pyrophosphate (PPi) and was only moderately inhibited by PPi. The breakdown of PPi was performed by a soluble pyrophosphatase. This enzyme was also purified and characterized. The pyrophosphatase hydrolyzed the major part of PPi (KM = 0.27 ± 0.05 mM) that was produced in the acetate activation reaction. -

Table S1. List of Oligonucleotide Primers Used
Table S1. List of oligonucleotide primers used. Cla4 LF-5' GTAGGATCCGCTCTGTCAAGCCTCCGACC M629Arev CCTCCCTCCATGTACTCcgcGATGACCCAgAGCTCGTTG M629Afwd CAACGAGCTcTGGGTCATCgcgGAGTACATGGAGGGAGG LF-3' GTAGGCCATCTAGGCCGCAATCTCGTCAAGTAAAGTCG RF-5' GTAGGCCTGAGTGGCCCGAGATTGCAACGTGTAACC RF-3' GTAGGATCCCGTACGCTGCGATCGCTTGC Ukc1 LF-5' GCAATATTATGTCTACTTTGAGCG M398Arev CCGCCGGGCAAgAAtTCcgcGAGAAGGTACAGATACGc M398Afwd gCGTATCTGTACCTTCTCgcgGAaTTcTTGCCCGGCGG LF-3' GAGGCCATCTAGGCCATTTACGATGGCAGACAAAGG RF-5' GTGGCCTGAGTGGCCATTGGTTTGGGCGAATGGC RF-3' GCAATATTCGTACGTCAACAGCGCG Nrc2 LF-5' GCAATATTTCGAAAAGGGTCGTTCC M454Grev GCCACCCATGCAGTAcTCgccGCAGAGGTAGAGGTAATC M454Gfwd GATTACCTCTACCTCTGCggcGAgTACTGCATGGGTGGC LF-3' GAGGCCATCTAGGCCGACGAGTGAAGCTTTCGAGCG RF-5' GAGGCCTGAGTGGCCTAAGCATCTTGGCTTCTGC RF-3' GCAATATTCGGTCAACGCTTTTCAGATACC Ipl1 LF-5' GTCAATATTCTACTTTGTGAAGACGCTGC M629Arev GCTCCCCACGACCAGCgAATTCGATagcGAGGAAGACTCGGCCCTCATC M629Afwd GATGAGGGCCGAGTCTTCCTCgctATCGAATTcGCTGGTCGTGGGGAGC LF-3' TGAGGCCATCTAGGCCGGTGCCTTAGATTCCGTATAGC RF-5' CATGGCCTGAGTGGCCGATTCTTCTTCTGTCATCGAC RF-3' GACAATATTGCTGACCTTGTCTACTTGG Ire1 LF-5' GCAATATTAAAGCACAACTCAACGC D1014Arev CCGTAGCCAAGCACCTCGgCCGAtATcGTGAGCGAAG D1014Afwd CTTCGCTCACgATaTCGGcCGAGGTGCTTGGCTACGG LF-3' GAGGCCATCTAGGCCAACTGGGCAAAGGAGATGGA RF-5' GAGGCCTGAGTGGCCGTGCGCCTGTGTATCTCTTTG RF-3' GCAATATTGGCCATCTGAGGGCTGAC Kin28 LF-5' GACAATATTCATCTTTCACCCTTCCAAAG L94Arev TGATGAGTGCTTCTAGATTGGTGTCggcGAAcTCgAGCACCAGGTTG L94Afwd CAACCTGGTGCTcGAgTTCgccGACACCAATCTAGAAGCACTCATCA LF-3' TGAGGCCATCTAGGCCCACAGAGATCCGCTTTAATGC RF-5' CATGGCCTGAGTGGCCAGGGCTAGTACGACCTCG -

Ruminal Microbiome-Host Crosstalk Stimulates the Development of the Ruminal Epithelium in a Lamb Model
Lin et al. Microbiome (2019) 7:83 https://doi.org/10.1186/s40168-019-0701-y RESEARCH Open Access Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model Limei Lin1,2†, Fei Xie1,2†, Daming Sun1,2, Junhua Liu1,2, Weiyun Zhu1,2 and Shengyong Mao1,2* Abstract Background: The development of the rumen is an important physiological challenge for young ruminants. Previous studies have shown that starter feeding can effectively facilitate the growth and development of the rumen in ruminants. However, the mechanism through which starter feeding stimulates the development of the rumen is not clear. Here, we performed an integrated analysis in ruminal microbiota and a host transcriptomic profile in a lamb model with the intervention of starter feed to understand the ruminal microbiome-host crosstalk in stimulating the development of the ruminal epithelium. Results: Decreased ruminal pH and increased acetate and butyrate concentrations in the rumen, followed by increasing rumen organ index, were observed in lambs supplemented with starter. Using metagenome sequencing in combination with 16S rRNA and 18S rRNA gene amplicon sequencing, the results showed the abundance of acetate-producing Mitsuokella spp., lactate-producing Sharpea spp., lactate-utilizing Megasphaera spp., and Entodinium spp. was enriched in rumen microbial communities in the starter-feed group. The abundances of genes involved in sugar degradation were decreased in starter-feed lambs, but the GH13 encoding α-amylase was obviously increased. Rumen epithelial transcriptome analysis revealed that seven differentially expressed genes, including MAPK1, PIK3CB, TNFSF10, ITGA6, SNAI2, SAV1,andDLG, related to the cell growth module were upregulated, and BAD’s promotion of cell death was downregulated. -
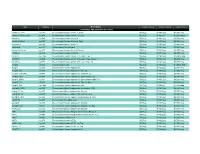
Prospec (Product
Name Catalog # Description Customer Price-A Customer Price-B Customer Price-C CYTOKINES AND GROWTH FACTORS Activin A, Active cyt-145 Recombinant Human Activin-A, Active $50/2µg $130/10µg $3,500/1mg Activin A, Plant-Active cyt-414 Recombinant Human Activin-A Active $50/1µg $130/5µg $1,500/100µg Activin A cyt-569 Recombinant Human Activin-A $50/2µg $130/10µg $2,700/1mg Activin A, Plant cyt-052 Recombinant Human Activin-A, Plant $50/2µg $130/10µg $4,800/1mg mActivin A cyt-146 Recombinant Mouse Activin-A $50/2µg $130/10µg $3,500/1mg rActivin A cyt-147 Recombinant Rat Activin-A $50/2µg $130/10µg $3,500/1mg Activin B, Active cyt-057 Recombinant Human Activin-B Active $50/2µg $130/10µg $5,200/1mg Activin B cyt-058 Recombinant Human Activin-B $50/2µg $130/10µg $4,800/1mg ACVR1 cyt-1140 Recombinant Human Activin A Receptor Type 1 $50/2µg $130/10µg $1,000/0.1mg ACVRL1 cyt-920 Recombinant Human Activin A Receptor Type II-Like 1 $50/2µg $130/10µg $5,200/1mg ACVR2A cyt-976 Recombinant Human Activin A Receptor Type 2A $50/2µg $130/10µg $5,200/1mg Acrp30 cyt-024 Human Adiponectin $50/2µg $130/10µg $1,000/0.1mg Acrp30 cyt-280 Recombinant Human Adiponectin $50/5µg $130/25µg $2,700/1mg Acrp30, His cyt-433 Recombinant Human Adiponectin, His tag $50/10µg $130/50µg $1,900/1mg Acrp30 (108-244) cyt-073 Recombinant Human Adiponectin (108-244 a.a.) $50/5µg $130/25µg $2,700/1mg Acrp30, HEK cyt-434 Recombinant Human Adiponectin glycosilated, HEK $50/2µg $130/10µg $4,680/1mg Acrp30, HMW cyt-764 Recombinant Human Adiponectin glycosilated, HMW Rich $50/2µg $130/10µg -

Prospec Product Catalog 2019
ProSpec Product Catalog 2019 QUALITY PROTEINS for scientific discoveries WE'VE GOT CHEMISTRY BRIGHTEN YOUR RESEARCH part for life sciences 6,000 proteins at your fingertips List by Molecule Name List by Molecule Name 제품별 자세한 설명은 www.prospecbio.com을 방문하세요. 부가세 별도 Molecule 제품번호 제품명 Size A / 가격(원) Size B / 가격(원) Size C / 가격(원) Name 1F8 Chagas cch-002 Recombinant 1F8 Chagas 5µg / 90,500 20µg / 199,000 1mg / 5,110,500 4-1BBL cyt-649 Recombinant Human 4-1BB Ligand, His Tag 5µg / 112,200 20µg / 220,800 1mg / 3,793,200 4-1BBL cyt-149 Recombinant Human 4-1BB Ligand 5µg / 90,500 20µg / 199,000 1mg / 3,771,400 4-1BBR cyt-916 Recombinant Mouse 4-1BB Receptor 1µg / 112,200 5µg / 220,800 50µg / 1,701,000 4-1BBR cyt-463 Recombinant Human 4-1BB Receptor 5µg / 90,500 20µg / 199,000 1mg / 3,771,400 4-1BBR cyt-137 Recombinant Human 4-1BB Receptor His Tag 5µg / 112,200 20µg / 220,800 1mg / 5,016,800 4-1BBR cyt-931 Recombinant Human 4-1BB Receptor sf9 2µg / 112,200 10µg / 220,800 1mg / 7,227,300 A2LD1 pro-172 Recombinant Human AIG2-Like Domain 1 2µg / 112,200 10µg / 220,800 1mg / 7,227,300 A2M pro-551 Human Alpha-2 Macroglobulin Protein 200µg / 90,500 1mg / 317,500 10mg / 2,389,800 Recombinant Human Alpha & Gamma-Adaptin Binding AAGAB pro-1479 5µg / 112,200 20µg / 220,800 1mg / 3,793,200 Protein Recombinant Human Adipogenesis Associated, Mth938 AAMDC pro-2116 5µg / 112,200 20µg / 220,800 1mg / 3,793,200 Domain Containing AARS enz-305 Recombinant Human Alanyl t-RNA Synthetase 5µg / 112,200 20µg / 220,800 1mg / 5,372,000 Recombinant Human Aminoadipate-Semialdehyde -

Acetate Kinase from Escherichia Coli (A7437)
SIGMA QUALITY CONTROL TEST ProductInformation PROCEDURE Enzymatic Assay of ACETATE KINASE1 (EC 2.7.2.1) PRINCIPLE: Acetate + ATP Acetate Kinase> Acetyl Phosphate + ADP ADP + PEP Pyruvate Kinase> ATP + Pyruvate Pyruvate + β-NADH Lactic Dehydrogenase> Lactate + β-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate ADP = Adenosine 5'-Diphosphate PEP = Phospho(enol)Pyruvate β-NADH = β-Nicotinamide Adenine Dinucleotide, Reduced Form β-NAD = β-Nicotinamide Adenine Dinucleotide, Oxidized Form CONDITIONS: T = 25EC, pH = 7.6, A340nm, Light path = 1 cm METHOD: Continuous Spectrophotometric Rate Determination REAGENTS: A. 100 mM Triethanolamine Buffer, pH 7.6 at 25EC. (Prepare 50 ml in deionized water using Triethanolamine Hydrochloride, Sigma Prod. No. T-1502. Adjust to pH 7.6 at 25EC with 1 M NaOH.) B. 1 M Sodium Acetate Solution (NaOAc) (Prepare 10 ml in deionized water using Sodium Acetate, Trihydrate, Sigma Prod. No. S-8625. PREPARE FRESH.) C. 91 mM Adenosine 5'-Triphosphate Solution (ATP) (Prepare 3 ml in deionized water using Adenosine 5'-Triphosphate, Disodium Salt, Sigma Prod. No. A-5394. PREPARE FRESH.) A7437 Page 1 of 4 SPACET01 Revised: 08/14/95 Enzymatic Assay of ACETATE KINASE1 (EC 2.7.2.1) REAGENTS: (continued) D. 56 mM Phospho(enol)pyruvate Solution (PEP) (Prepare 1.5 ml in deionized water using Phospho(enol)pyruvate, Mono(cyclohexylammonium) Salt, Sigma Prod. No. P-3637. PREPARE FRESH.) E. 200 mM Magnesium Chloride Solution (MgCl2) (Prepare 5 ml in deionized water using Magnesium Chloride, Hexahydrate, Sigma Prod. No. M-0250.) F. 6.4 mM β-Nicotinamide Adenine Dinucleotide, Reduced Form Solution (β-NADH) (Dissolve the contents of one 5 mg vial of β-Nicotinamide Adenine Dinucleotide, Reduced Form, Disodium Salt, Sigma Stock No. -

Modulation of Acetate Utilization in Komagataella Phaffii by Metabolic
Xu et al. Biotechnol Biofuels (2019) 12:61 https://doi.org/10.1186/s13068-019-1404-0 Biotechnology for Biofuels RESEARCH Open Access Modulation of acetate utilization in Komagataella phafi by metabolic engineering of tolerance and metabolism Qin Xu1, Chenxiao Bai1, Yiqi Liu1, Lili Song1, Lin Tian1, Yunfeng Yan1, Jinfeng Zhou1, Xiangshan Zhou1, Yuanxing Zhang1,2 and Menghao Cai1* Abstract Background: Acetate, an economical industrial chemical, which is also the precursor of acetyl-CoA, could serve as an alternative substrate for biomanufacturing. This nontraditional substrate can be widely produced from syngas via hydrolysis or pyrolysis of the cellulosic biomass, chemical or microbial catalysis, anaerobic fermentation in treated wastewater, etc. However, the toxicity of acetate to microorganisms has held back its utilization, especially for the eukaryotes that are good hosts for production of complicated pharmaceuticals or chemicals. This study seeks to improve acetate utilization in a widely used yeast host, Komagataella phafi (previously Pichia pastoris), by metabolic engineering of acetate tolerance, transport, and metabolism. Results: A kinase-defcient library of K. phafi was frstly used to screen acetate-resistant kinases. The HRK1 knockout strain was sensitive to acetate and overexpression of this gene improved acetate tolerance and cell growth of the strain. Also, overexpression of HRK1 caused a 55% productivity improvement of acetyl-CoA-dependent 6-methylsalicylic acid (6-MSA). However, activation of Hrk1 on membrane H( )-ATPase Pma1 seemed not to work in the engineered strain. Acetate transporter gene ScFPS1* was further overexpressed,+ despite of not improving 6-MSA biosynthesis. To enhance acetate metabolism, acetyl-CoA synthesizing related genes, yeast PpACS1, ScACS1*, and E. -

How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication
REVIEW published: 04 March 2019 doi: 10.3389/fcimb.2019.00042 How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication Wolfgang Eisenreich 1*, Thomas Rudel 2, Jürgen Heesemann 3 and Werner Goebel 3* 1 Chair of Biochemistry, Department of Chemistry, Technische Universität München, Garching, Germany, 2 Chair of Microbiology, Biocenter, University of Würzburg, Würzburg, Germany, 3 Max von Pettenkofer-Institute, Ludwig Maximilian University of Munich, Munich, Germany Edited by: Saliha Durmu ¸s, Gebze Technical University, Turkey Viruses and intracellular bacterial pathogens (IBPs) have in common the need of suitable Reviewed by: host cells for efficient replication and proliferation during infection. In human infections, Fernando Navarro-Garcia, the cell types which both groups of pathogens are using as hosts are indeed quite similar Centro de Investigación y de Estudios and include phagocytic immune cells, especially monocytes/macrophages (MOs/MPs) Avanzados (CINVESTAV), Mexico Wen-Ching Wang, and dendritic cells (DCs), as well as nonprofessional phagocytes, like epithelial cells, National Tsing Hua University, Taiwan fibroblasts and endothelial cells. These terminally differentiated cells are normally in a *Correspondence: metabolically quiescent state when they are encountered by these pathogens during Wolfgang Eisenreich [email protected] infection. This metabolic state of the host cells does not meet the extensive need Werner Goebel for nutrients required for efficient intracellular replication of viruses and especially IBPs [email protected] which, in contrast to the viral pathogens, have to perform their own specific intracellular Specialty section: metabolism to survive and efficiently replicate in their host cell niches.