Metastatic Spread from Abdominal Tumor Cells to Parathymic Lymph Nodes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter 2 ROLE of LYMPHOSCINTIGRAPHY for SELECTIVE SENTINEL LYMPHADENECTOMY
Chapter 2 ROLE OF LYMPHOSCINTIGRAPHY FOR SELECTIVE SENTINEL LYMPHADENECTOMY Roger F. Uren, Robert B. Howman-Giles, David Chung, John F. Thompson* Nuclear Medicine and Diagnostic Ultrasound, RPAH Medical Centre and Discipline oj Medicine, The University of Sydney, Sydney, NSW, Australia and The Sydney Melanoma Unit, Royal Prince Alfred Hospital, Camperdown, NSW and Discipline of Surgery*, The University of Sydney, Sydney, NSW, Australia Abstract: An essential prerequisite for a successful sentinel node biopsy (SNB) procedure is an accurate map of the pattern of lymphatic drainage from the primary tumor site. The role of lymphoscintigraphy(LS) in SNB is to provide such a map in each patient. This map should indicate not only the location of all sentinel nodes but also the number of SNs at each location. Such mapping can be achieved using 99mTc-labeled small particle radiocolloids, high- resolution collimators with minimal septal penetration, and imaging protocols that detect all SNs in every patient regardless of their location. This is especially important in melanoma patients, since high-quality LS can identify the actual lymphatic collecting vessels as they drain into each SN. The SN is not always found in the nearest node field and is best defined as "any lymph node receiving direct lymphatic drainage from a primary tumor site." Reliable clinical prediction of lymphatic drainage from the skin or breast is not possible. Patterns of lymphatic drainage from the skin are highly variable from patient to patient, even from the same area of the skin. Unexpected lymphatic drainage has been found from the skin of the back to SNs in the triangular intermuscular space and in some patients through the posterior body wall to SNs in the para-aortic, paravertebral, and retroperitoneal areas. -

Human Anatomy As Related to Tumor Formation Book Four
SEER Program Self Instructional Manual for Cancer Registrars Human Anatomy as Related to Tumor Formation Book Four Second Edition U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutesof Health SEER PROGRAM SELF-INSTRUCTIONAL MANUAL FOR CANCER REGISTRARS Book 4 - Human Anatomy as Related to Tumor Formation Second Edition Prepared by: SEER Program Cancer Statistics Branch National Cancer Institute Editor in Chief: Evelyn M. Shambaugh, M.A., CTR Cancer Statistics Branch National Cancer Institute Assisted by Self-Instructional Manual Committee: Dr. Robert F. Ryan, Emeritus Professor of Surgery Tulane University School of Medicine New Orleans, Louisiana Mildred A. Weiss Los Angeles, California Mary A. Kruse Bethesda, Maryland Jean Cicero, ART, CTR Health Data Systems Professional Services Riverdale, Maryland Pat Kenny Medical Illustrator for Division of Research Services National Institutes of Health CONTENTS BOOK 4: HUMAN ANATOMY AS RELATED TO TUMOR FORMATION Page Section A--Objectives and Content of Book 4 ............................... 1 Section B--Terms Used to Indicate Body Location and Position .................. 5 Section C--The Integumentary System ..................................... 19 Section D--The Lymphatic System ....................................... 51 Section E--The Cardiovascular System ..................................... 97 Section F--The Respiratory System ....................................... 129 Section G--The Digestive System ......................................... 163 Section -

Lymphatic Drainage of the Breast: from Theory to Surgical Practice
Int. J. Morphol., 27(3):873-878, 2009. Lymphatic Drainage of the Breast: from Theory to Surgical Practice Drenaje Linfático de la Mama: desde la Teoría a la Práctica Quirúrgica *José Humberto Tavares Guerreiro Fregnani & **José Rafael Macéa FREGNANI, J. H. T. G. & MACÉA, J. R. Lymphatic drainage of the breast: from theory to surgical practice. Int. J. Morphol., 27(3):873-878, 2009. SUMMARY: Until recently, complete removal of axillary lymph nodes was performed as part of the treatment of breast cancer. Sentinel lymph node biopsy (SLNB) in selected cases has reduced the number of cases of wide axillary dissection and the related morbidity. Knowledge of breast lymphatic drainage is essential for understanding the principles behind SLNB and also for performing safe and correct axillary lymphonodectomy. This paper describes in detail the anatomical issues relating to breast lymphatic drainage and the correlated axillary and extra-axillary lymph nodes. In addition, it shows the application of this theoretical knowledge to surgical practice, especially with regard to SLNB and lymphonodectomy. The surgical nomenclature is compared with the current International Anatomical Terminology. KEY WORDS: Lymphatic drainage, Sentinel lymph node biopsy, Breast cancer. INTRODUCTION Breast cancer is the most frequent type of tumor changes to the sensitivity of the upper limb, posterior scapular among women, accounting for approximately one quarter dislocation (winged scapula syndrome), brachial plexus of all tumors in women. It has been estimated that more than lesions, axillary vessel thrombosis and lesions, skin necrosis one million new cases occur worldwide annually. Breast and pectoral muscle atrophy, among others (Torresan et al., cancer is responsible for significant morbidity and mortality 2002; Kim et al., 2006). -

The Lymphoid System
LYMPHATIC SYSTEM MUDr. Hisham El Falougy, PhD. [email protected] Lymphoid cells Lymphoid organs: primary and secondary Lymphoid vessels lymph The lymphoid cells B lymphocytes Plasma cells Humeral immunity (IgG, IgA, IgM, IgD, IgE) B memory cells The lymphoid cells T lymphocytes Cellular imunity Cytotoxic cells Helper cells Supressor cells T memory cells The lymphoid cells Antigen-presenting cells Macrophages Epidermal Langerhans cells Dendritic cells of lymphoid organs M cells The primary lymphoid organs Bone marrow Red bone marrow Yellow bone marrow Thymus The secondary lymphoid organs Spleen Lymph nodes Unencapsulated lymphoid tissue Tonsils LYMPHATIC SYSTEM FUNCTIONS: TRANSPORTS EXCESS INTERSTITIAL FLUID ABSORBS AND TRANSPORTS FAT FROM INTESTINE IMMUNOLOGICAL FUNCTION LYMPH AND LYMPH CAPILLARIES LYMPH CAPILLARIES SMALLEST LYMPHATIC VESSELS CLOSED-ENDED TUBES FORM NETWORK IN THE INTERCELLULAR SPACES LACTEALS (SMALL INTESTINE) LYMPH AND LYMPH CAPILLARIES LYMPH CAPILLARIES ENDOTHELIUM LACK A BASAL LAMINA PERMAEABLE TO LARGER MOLECULES LYMPH AND LYMPH CAPILLARIES LYMPH CAPILLARIES ABSENT FROM: AVASCULAR STRUCTURES CNS BONE MARROW VERY FEW IN ENDOMYSIUM OF SKELETAL MUSCLES LYMPH AND LYMPH CAPILLARIES LYMPH FILTRATE OF PLASMA CLEAR AND COLOURLESS DENSE AND MILKY CHYLE LYMPHATIC VESSELS LYMPH CAPILLARIES JOIN INTO LARGER LYMPHATIC VESSELS PASS TO LOCAL OR REMOTE LYMPH NODES REPAIR EASILY Lymphatic vessels and lymph Vasa lymphocapillaria Rete lymphocapillare Collectores lymphatici -

Anatomy and Physiology in Relation to Compression of the Upper Limb and Thorax
Clinical REVIEW anatomy and physiology in relation to compression of the upper limb and thorax Colin Carati, Bren Gannon, Neil Piller An understanding of arterial, venous and lymphatic flow in the upper body in normal limbs and those at risk of, or with lymphoedema will greatly improve patient outcomes. However, there is much we do not know in this area, including the effects of compression upon lymphatic flow and drainage. Imaging and measuring capabilities are improving in this respect, but are often expensive and time-consuming. This, coupled with the unknown effects of individual, diurnal and seasonal variances on compression efficacy, means that future research should focus upon ways to monitor the pressure delivered by a garment, and its effects upon the fluids we are trying to control. More is known about the possible This paper will describe the vascular Key words effects of compression on the anatomy of the upper limb and axilla, pathophysiology of lymphoedema when and will outline current understanding of Anatomy used on the lower limbs (Partsch and normal and abnormal lymph drainage. It Physiology Junger, 2006). While some of these will also explain the mechanism of action Lymphatics principles can be applied to guide the use of compression garments and will detail Compression of compression on the upper body, it is the effects of compression on fluid important that the practitioner is movement. knowledgeable about the anatomy and physiology of the upper limb, axilla and Vascular drainage of the upper limb thorax, and of the anatomical and vascular It is helpful to have an understanding of Little evidence exists to support the differences that exist between the upper the vascular drainage of the upper limb, use of compression garments in the and lower limb, so that the effects of these since the lymphatic drainage follows a treatment of lymphoedema, particularly differences can be considered when using similar course (Figure 1). -
Anatomy of the Breast Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of the Breast Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives By the end of the lecture, the student should be able to: ✓ Describe the shape and position of the female breast. ✓ Describe the structure of the mammary gland. ✓ List the blood supply of the female breast. ✓ Describe the lymphatic drainage of the female breast. ✓ Describe the applied anatomy in the female breast. Highly recommended Introduction 06:26 Overview of the breast: • The breast (consists of mammary glands + associated skin & Extra connective tissue) is a gland made up of lobes arranged radially .around the nipple (شعاعيا) • Each lobe is further divided into lobules. Between the lobes and lobules we have fat & ligaments called ligaments of cooper • These ligaments attach the skin to the muscle (beneath the breast) to give support to the breast. in shape (مخروطي) *o Shape: it is conical o Position: It lies in superficial fascia of the front of chest. * o Parts: It has a: 1. Base lies on muscles, (حلمة الثدي) Apex nipple .2 3. Tail extend into axilla Extra Position of Female Breast (حلقة ملونة) Base Nipple Areola o Extends from 2nd to 6th ribs. o It extends from the lateral margin of sternum medially to the midaxillary line laterally. o It has no capsule. o It lies on 3 muscles: • 2/3 of its base on (1) pectoralis major* Extra muscle, • inferolateral 1/3 on (2) Serratus anterior & (3) External oblique muscles (muscle of anterior abdominal wall). o Its superolateral part sends a process into the axilla called the axillary tail or axillary process. -
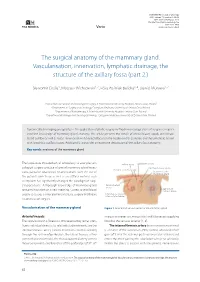
The Surgical Anatomy of the Mammary Gland. Vascularisation, Innervation, Lymphatic Drainage, the Structure of the Axillary Fossa (Part 2.)
NOWOTWORY Journal of Oncology 2021, volume 71, number 1, 62–69 DOI: 10.5603/NJO.2021.0011 © Polskie Towarzystwo Onkologiczne ISSN 0029–540X Varia www.nowotwory.edu.pl The surgical anatomy of the mammary gland. Vascularisation, innervation, lymphatic drainage, the structure of the axillary fossa (part 2.) Sławomir Cieśla1, Mateusz Wichtowski1, 2, Róża Poźniak-Balicka3, 4, Dawid Murawa1, 2 1Department of General and Oncological Surgery, K. Marcinkowski University Hospital, Zielona Gora, Poland 2Department of Surgery and Oncology, Collegium Medicum, University of Zielona Gora, Poland 3Department of Radiotherapy, K. Marcinkowski University Hospital, Zielona Gora, Poland 4Department of Urology and Oncological Urology, Collegium Medicum, University of Zielona Gora, Poland Dynamically developing oncoplasty, i.e. the application of plastic surgery methods in oncological breast surgeries, requires excellent knowledge of mammary gland anatomy. This article presents the details of arterial blood supply and venous blood outflow as well as breast innervation with a special focus on the nipple-areolar complex, and the lymphatic system with lymphatic outflow routes. Additionally, it provides an extensive description of the axillary fossa anatomy. Key words: anatomy of the mammary gland The large-scale introduction of oncoplasty to everyday on- axillary artery subclavian artery cological surgery practice of partial mammary gland resec- internal thoracic artery thoracic-acromial artery tions, partial or total breast reconstructions with the use of branches to the mammary gland the patient’s own tissue as well as an artificial material such as implants has significantly changed the paradigm of surgi- cal procedures. A thorough knowledge of mammary gland lateral thoracic artery superficial anatomy has taken on a new meaning. -

Patient Guide to Lymphedema and Breast Cancer
Patient Guide to Lymphedema and Breast Cancer Marcia Pearl, PT, PhD, CLT Jill Binkley, PT, MSc Cathy Furbish, PT, DPT Overview and Facts: Definition: Lymphedema is an abnormal accumulation of lymphatic fluid in the tissues that causes swelling. It can occur in the arm, trunk, abdomen or breast following breast cancer treatment. Lymphedema is the result of damaged or blocked lymphatic vessels caused by surgery, radiation therapy, injury, limb paralysis, infection, or an inflammatory condition. Surgery combined with radiation therapy for breast cancer is the most common cause of arm lymphedema for women in the United States The Lymph System: The lymph system is a one-way drainage route designed to rid the tissues of unwanted material and excess fluid. The lymphatic system plays a large role in immune function and circulation. It consists of lymph vessels located just under the skin. Everywhere where you have a blood vessel you have a lymph vessel, from the top of your head to the tips of your toes. The lymph fluid is "pushed" through the lymph system by the compression of surrounding muscles. The lymph vessels empty into lymph nodes. The lymph nodes are found along the lymph vessel, they look like a string of pearls. There are approximately 600-700 lymph nodes throughout the body. The lymph nodes are gathered in clusters in your armpit, groin and neck. As the lymph vessels move fluid out of the tissues, waste products, bacteria, dead cells and large protein molecules are collected. The waste products are carried to the lymph nodes and broken down and eliminated, while the protein rich fluid is transported back to the heart where it rejoins the circulation. -

Lecture №3. METHODS of RESEARCH of the PATIENT
Lecture №3. METHODS OF RESEARCH OF THE PATIENT. PALPATION. GENERAL PRINCIPLES, METHODS. PALPATION OF THE THORAX, HEART, ABDOMEN, LIVER, SPLEEN, KIDNEY, LYMPH NODES, THYROID GLAND. Palpation (lat. palpatio - feeling) is a clinical method of direct examination of a patient with the help of touch to study the physical properties of tissues and organs, the topographical relationships between them, and their soreness. This method of research has been known since Hippocrates, but until the XIX century, the use of palpation was limited to the study of the condition of the skin, joints, bones and the properties of the pulse. From the middle of the XIX century, clinical practice included the study of vocal tremor and apical heart beat (Laennek, Piorri, Skoda). Systematic palpation of the abdominal cavity became possible only from the end of the last century and the beginning of this century after the publication of the works of S.Botkin, F. Glenar, V.Obraztsova and N.Strazhesko. Further development of palpation of the abdominal cavity led to the creation of a harmonious theory of deep, sliding, topographical, methodological palpation. The physiological basis of palpation is the sense of touch. If the density of the palpable organ is greater than the density of the medium, a tactile sensation arises when one probes an organ through an intermediate medium. It appears when the consistency of the tissues under the fingers changes or when the movement is blocked. it is necessary to press the organ under examination to a dense basis (the pelvic bone, the palm of the doctor's palm resting under the waist of the patient) to palpate a relatively soft body (gut). -

Female Breast
FEMALE BREAST PROF. Saeed Abuel Makarem & DR.SANAA AL-SHAARAWI OBJECTIVES • By the end of the lecture, the student should be able to: • Describe the shape and position of the female breast. • Describe the structure of the mammary gland. • List the blood supply of the female breast. • Describe the lymphatic drainage of the female breast. • Describe the applied anatomy in the female breast. Parts, Shape & position of the Gland • It is conical in shape. • It lies in superficial fascia of the front of chest. • It has a base, apex and tail. • Its base extends from 2nd to 6th ribs. • It extends from the sternum to the midaxillary line laterally. • It has no capsule. SHAPE AND POSITION OF FEMALE BREAST • 2/3 of its base lies on the pectoralis major muscle, while its inferolateral 1/3 lies on: • Serratus anterior & • External oblique muscles. • Its superolateral part sends a process into the axilla called the axillary tail or axillary process. SHAPE AND POSITION OF FEMALE BREAST • Nipple: • It is a conical eminence that projects forwards from the anterior surface of the breast. • The nipple lies opposite 4th intercostal space. • It carries 15-20 narrow pores of the lactiferous ducts. • Areola : • It is a dark pink brownish circular area of skin that surrounds the nipple. • The subcutaneous tissues of nipple & areola are devoid of fat. STRUCTURE OF MAMMARY GLAND • It is non capsulated gland. • It consists of lobes and lobules which are embedded in the subcutaneous fatty tissue of superficial fascia. • It has fibrous strands (ligaments of cooper) which connect the skin with deep fascia of pectoralis major. -

Radionuclide Lymphoscintigraphy in the Evaluation of Lymphedema*
CONTINUING EDUCATION The Third Circulation: Radionuclide Lymphoscintigraphy in the Evaluation of Lymphedema* Andrzej Szuba, MD, PhD1; William S. Shin1; H. William Strauss, MD2; and Stanley Rockson, MD1 1Division of Cardiovascular Medicine, Stanford University School of Medicine, Stanford, California; and 2Division of Nuclear Medicine, Stanford University School of Medicine, Stanford, California all. Lymphedema results from impaired lymphatic transport Lymphedema—edema that results from chronic lymphatic in- caused by injury to the lymphatics, infection, or congenital sufficiency—is a chronic debilitating disease that is frequently abnormality. Patients often suffer in silence when their misdiagnosed, treated too late, or not treated at all. There are, primary physician or surgeon suggests that the problem is however, effective therapies for lymphedema that can be im- plemented, particularly after the disorder is properly diagnosed mild and that little can be done. Fortunately, there are and characterized with lymphoscintigraphy. On the basis of the effective therapies for lymphedema that can be imple- lymphoscintigraphic image pattern, it is often possible to deter- mented, particularly after the disorder is characterized with mine whether the limb swelling is due to lymphedema and, if so, lymphoscintigraphy. whether compression garments, massage, or surgery is indi- At the Stanford Lymphedema Center, about 200 new cated. Effective use of lymphoscintigraphy to plan therapy re- cases of lymphedema are diagnosed each year (from a quires an understanding of the pathophysiology of lymphedema and the influence of technical factors such as selection of the catchment area of about 500,000 patients). Evidence that the radiopharmaceutical, imaging times after injection, and patient disease is often overlooked by physicians caring for the activity after injection on the images. -

Patterns of Lymphatic Drainage from the Skin in Patients with Melanoma*
CONTINUING EDUCATION Patterns of Lymphatic Drainage from the Skin in Patients with Melanoma* Roger F. Uren, MD1–3; Robert Howman-Giles, MD1–3; and John F. Thompson, MD3,4 1Nuclear Medicine and Diagnostic Ultrasound, RPAH Medical Centre, Sydney, New South Wales, Australia; 2Department of Medicine, University of Sydney, Sydney, New South Wales, Australia; 3Sydney Melanoma Unit, Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia; and 4Department of Surgery, University of Sydney, Sydney, New South Wales, Australia Key Words: lymphatic drainage; skin melanoma An essential prerequisite for a successful sentinel lymph node J Nucl Med 2003; 44:570–582 biopsy (SLNB) procedure is an accurate map of the pattern of lymphatic drainage from the primary tumor site in each patient. In melanoma patients, mapping requires high-quality lympho- scintigraphy, which can identify the actual lymphatic collecting vessels as they drain into the sentinel lymph nodes. Small- This article has been prepared to complement the review particle radiocolloids are needed to achieve this goal, and im- of sentinel lymph node biopsy (SLNB) in melanoma written aging protocols must be adapted to ensure that all true sentinel by Mariani et al. (1) and published in 2002. That review nodes, including those in unexpected locations, are found in provided a detailed account of the technical aspects of every patient. Clinical prediction of lymphatic drainage from the SLNB in melanoma. In this article, we concentrate on the skin is not possible. The old clinical guidelines based on common and less common patterns of lymphatic drainage Sappey’s lines therefore should be abandoned. Patterns of that are seen in melanoma patients.