Bay Du Nord Development Project Environmental Impact Statement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Goh, Ee Von (2018) the Status of Fish in Malaysian Diets and Potential Barriers to Increasing Consumption of Farmed Species
THE STATUS OF FISH IN MALAYSIAN DIETS AND POTENTIAL BARRIERS TO INCREASING CONSUMPTION OF FARMED SPECIES Ee Von Goh Thesis submitted to the University of Nottingham Malaysia Campus for the degree of Doctor of Philosophy 2018 Abstract………………………………………………………………………………………………………………………………………….i Dedications and Acknowledgements……………………………………………………………………………………………..ii List of Tables…………………………………………………………………………………………………………………………………iii List of Figures…………………………………………………………………………………………………………………………………v List of Abbreviations.…………………………………………………………………………………………………………………….vi Chapter 1: Introduction 1.1 The Concept of Sustainable Diet for Human Health…………………………………………………………1 1.1.1 Indicators for Sustainable Consumption…………………………………………………………..3 1.2 The Importance of Fish in the Livelihood of Malaysian ……………………………………………………5 1.3 Fish Consumption Pattern in Malaysia………………………………............................................... 8 1.4 Fish Purchasing Behaviour of Malaysian…………………………………………………………………………. 9 1.5 Status of Malaysian Fishery Industry……………………………………………………………………………. 12 1.5.1 Marine Capture Fisheries……………………………………………………………………………… 12 1.5.2 Aquaculture……………………………………………………………………………………………………13 1.5.3 Post-Harvest Utilisation………………………………………………………………………………… 14 1.5.4 Assessing the Sustainability of Fishery Production……………………………………….. 15 1.5.5 The (Un)Sustainability of The Wild Fish Supply……………………………………………… 16 1.5.6 General Perceptions of Fish Farming and Farmed Fish……………………………………20 1.6 Stakeholders’ Roles in Fish Sustainability……………………………………………………………………… 23 1.7 Addressing -

UN1VERSITY of Hawal'l LIBRARY
UN1VERSITY OF HAWAl'l LIBRARY SYMBIONT-INDUCED CHANGES IN HOST GENE EXPRESSION: THE SQUID VmRIO SYMBIOSIS A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI'I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOMEDICAL SCIENCES (CELL AND MOLECULAR BIOLOGy) DECEMBER 2003 By Jennifer Loraine Kimbell Dissertation Committee: Margaret McFall-Ngai, Chairperson Edward Ruby Tom Humphreys AlanLau Katalin Csiszar Karen Glanz ABSTRACT All animals exist in lifelong relations with a complement ofbacteria. Because of the ubiquity ofthese symbioses as well as the derived biomedical applications, the study ofboth beneficial and pathogenic host-microbe associations has long been established. The monospecific light organ association between the Hawaiian sepiolid squid Euprymrw se%pes and the marine luminous bacterium Vibrio fiseheri has been used as a experimental model for the study ofthe most common type ofanimal-bacterial interaction, i.e., the association ofcoevolved Gram-negative bacteria with the extracellular apical surfaces ofpolarized epithelia. A fundamental step for understanding the mechanisms ofhost-symbiont associations lies in defining the genetic components involved; specifically defining changes in host gene expression. The studies presented in this dissertation identify and characterize V. fiseheri-induced changes in host gene expression at both the transcript and protein level. iii TABLE OF CONTENTS Abstract. ...................................... .......................................... -
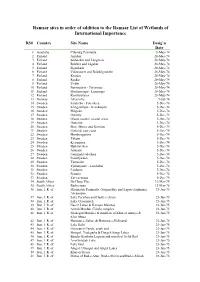
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Taxonomy and Diversity of the Sponge Fauna from Walters Shoal, a Shallow Seamount in the Western Indian Ocean Region
Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region By Robyn Pauline Payne A thesis submitted in partial fulfilment of the requirements for the degree of Magister Scientiae in the Department of Biodiversity and Conservation Biology, University of the Western Cape. Supervisors: Dr Toufiek Samaai Prof. Mark J. Gibbons Dr Wayne K. Florence The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the NRF. December 2015 Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region Robyn Pauline Payne Keywords Indian Ocean Seamount Walters Shoal Sponges Taxonomy Systematics Diversity Biogeography ii Abstract Taxonomy and diversity of the sponge fauna from Walters Shoal, a shallow seamount in the Western Indian Ocean region R. P. Payne MSc Thesis, Department of Biodiversity and Conservation Biology, University of the Western Cape. Seamounts are poorly understood ubiquitous undersea features, with less than 4% sampled for scientific purposes globally. Consequently, the fauna associated with seamounts in the Indian Ocean remains largely unknown, with less than 300 species recorded. One such feature within this region is Walters Shoal, a shallow seamount located on the South Madagascar Ridge, which is situated approximately 400 nautical miles south of Madagascar and 600 nautical miles east of South Africa. Even though it penetrates the euphotic zone (summit is 15 m below the sea surface) and is protected by the Southern Indian Ocean Deep- Sea Fishers Association, there is a paucity of biodiversity and oceanographic data. -

Northern Wolffish,Anarhichas Denticulatus
COSEWIC Assessment and Status Report on the Northern Wolffish Anarhichas denticulatus in Canada THREATENED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Northern Wolffish Anarhichas denticulatus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 41 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm) Previous report(s): COSEWIC. 2001. COSEWIC assessment and status report on the northern wolffish Anarhichas denticulatus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 21 pp. (www.sararegistry.gc.ca/status/status_e.cfm) O’Dea, N.R., and R.L. Haedrich. 2001. COSEWIC status report on the northern wolffish Anarhichas denticulatus in Canada, in COSEWIC assessment and status report on the northern wolffish Anarhichas denticulatus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-21 pp. Production note: COSEWIC would like to acknowledge Red Méthot for writing the status report on the Northern Wolffish, Anarhichas denticulatus in Canada, prepared under contract with Environment Canada. The report was overseen and edited by John Reynolds, COSEWIC Marine Fishes Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Loup à tête large (Anarhichas denticulatus) au Canada. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Appendix: Some Important Early Collections of West Indian Type Specimens, with Historical Notes
Appendix: Some important early collections of West Indian type specimens, with historical notes Duchassaing & Michelotti, 1864 between 1841 and 1864, we gain additional information concerning the sponge memoir, starting with the letter dated 8 May 1855. Jacob Gysbert Samuel van Breda A biography of Placide Duchassaing de Fonbressin was (1788-1867) was professor of botany in Franeker (Hol published by his friend Sagot (1873). Although an aristo land), of botany and zoology in Gent (Belgium), and crat by birth, as we learn from Michelotti's last extant then of zoology and geology in Leyden. Later he went to letter to van Breda, Duchassaing did not add de Fon Haarlem, where he was secretary of the Hollandsche bressin to his name until 1864. Duchassaing was born Maatschappij der Wetenschappen, curator of its cabinet around 1819 on Guadeloupe, in a French-Creole family of natural history, and director of Teyler's Museum of of planters. He was sent to school in Paris, first to the minerals, fossils and physical instruments. Van Breda Lycee Louis-le-Grand, then to University. He finished traveled extensively in Europe collecting fossils, especial his studies in 1844 with a doctorate in medicine and two ly in Italy. Michelotti exchanged collections of fossils additional theses in geology and zoology. He then settled with him over a long period of time, and was received as on Guadeloupe as physician. Because of social unrest foreign member of the Hollandsche Maatschappij der after the freeing of native labor, he left Guadeloupe W etenschappen in 1842. The two chief papers of Miche around 1848, and visited several islands of the Antilles lotti on fossils were published by the Hollandsche Maat (notably Nevis, Sint Eustatius, St. -

A Soft Spot for Chemistry–Current Taxonomic and Evolutionary Implications of Sponge Secondary Metabolite Distribution
marine drugs Review A Soft Spot for Chemistry–Current Taxonomic and Evolutionary Implications of Sponge Secondary Metabolite Distribution Adrian Galitz 1 , Yoichi Nakao 2 , Peter J. Schupp 3,4 , Gert Wörheide 1,5,6 and Dirk Erpenbeck 1,5,* 1 Department of Earth and Environmental Sciences, Palaeontology & Geobiology, Ludwig-Maximilians-Universität München, 80333 Munich, Germany; [email protected] (A.G.); [email protected] (G.W.) 2 Graduate School of Advanced Science and Engineering, Waseda University, Shinjuku-ku, Tokyo 169-8555, Japan; [email protected] 3 Institute for Chemistry and Biology of the Marine Environment (ICBM), Carl-von-Ossietzky University Oldenburg, 26111 Wilhelmshaven, Germany; [email protected] 4 Helmholtz Institute for Functional Marine Biodiversity, University of Oldenburg (HIFMB), 26129 Oldenburg, Germany 5 GeoBio-Center, Ludwig-Maximilians-Universität München, 80333 Munich, Germany 6 SNSB-Bavarian State Collection of Palaeontology and Geology, 80333 Munich, Germany * Correspondence: [email protected] Abstract: Marine sponges are the most prolific marine sources for discovery of novel bioactive compounds. Sponge secondary metabolites are sought-after for their potential in pharmaceutical applications, and in the past, they were also used as taxonomic markers alongside the difficult and homoplasy-prone sponge morphology for species delineation (chemotaxonomy). The understanding Citation: Galitz, A.; Nakao, Y.; of phylogenetic distribution and distinctiveness of metabolites to sponge lineages is pivotal to reveal Schupp, P.J.; Wörheide, G.; pathways and evolution of compound production in sponges. This benefits the discovery rate and Erpenbeck, D. A Soft Spot for yield of bioprospecting for novel marine natural products by identifying lineages with high potential Chemistry–Current Taxonomic and Evolutionary Implications of Sponge of being new sources of valuable sponge compounds. -

7. Index of Scientific and Vernacular Names
Cephalopods of the World 249 7. INDEX OF SCIENTIFIC AND VERNACULAR NAMES Explanation of the System Italics : Valid scientific names (double entry by genera and species) Italics : Synonyms, misidentifications and subspecies (double entry by genera and species) ROMAN : Family names ROMAN : Scientific names of divisions, classes, subclasses, orders, suborders and subfamilies Roman : FAO names Roman : Local names 250 FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1 A B Acanthosepion pageorum .....................118 Babbunedda ................................184 Acanthosepion whitleyana ....................128 bandensis, Sepia ..........................72, 138 aculeata, Sepia ............................63–64 bartletti, Blandosepia ........................138 acuminata, Sepia..........................97,137 bartletti, Sepia ............................72,138 adami, Sepia ................................137 bartramii, Ommastrephes .......................18 adhaesa, Solitosepia plangon ..................109 bathyalis, Sepia ..............................138 affinis, Sepia ...............................130 Bathypolypus sponsalis........................191 affinis, Sepiola.......................158–159, 177 Bathyteuthis .................................. 3 African cuttlefish..............................73 baxteri, Blandosepia .........................138 Ajia-kouika .................................. 115 baxteri, Sepia.............................72,138 albatrossae, Euprymna ........................181 belauensis, Nautilus .....................51,53–54 -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

Social, Economic and Cultural Overview of Western Newfoundland and Southern Labrador
Social, Economic and Cultural Overview of Western Newfoundland and Southern Labrador ii Oceans, Habitat and Species at Risk Publication Series, Newfoundland and Labrador Region No. 0008 March 2009 Revised April 2010 Social, Economic and Cultural Overview of Western Newfoundland and Southern Labrador Prepared by 1 Intervale Associates Inc. Prepared for Oceans Division, Oceans, Habitat and Species at Risk Branch Fisheries and Oceans Canada Newfoundland and Labrador Region2 Published by Fisheries and Oceans Canada, Newfoundland and Labrador Region P.O. Box 5667 St. John’s, NL A1C 5X1 1 P.O. Box 172, Doyles, NL, A0N 1J0 2 1 Regent Square, Corner Brook, NL, A2H 7K6 i ©Her Majesty the Queen in Right of Canada, 2011 Cat. No. Fs22-6/8-2011E-PDF ISSN1919-2193 ISBN 978-1-100-18435-7 DFO/2011-1740 Correct citation for this publication: Fisheries and Oceans Canada. 2011. Social, Economic and Cultural Overview of Western Newfoundland and Southern Labrador. OHSAR Pub. Ser. Rep. NL Region, No.0008: xx + 173p. ii iii Acknowledgements Many people assisted with the development of this report by providing information, unpublished data, working documents, and publications covering the range of subjects addressed in this report. We thank the staff members of federal and provincial government departments, municipalities, Regional Economic Development Corporations, Rural Secretariat, nongovernmental organizations, band offices, professional associations, steering committees, businesses, and volunteer groups who helped in this way. We thank Conrad Mullins, Coordinator for Oceans and Coastal Management at Fisheries and Oceans Canada in Corner Brook, who coordinated this project, developed the format, reviewed all sections, and ensured content relevancy for meeting GOSLIM objectives. -

Special Issue3.7 MB
Volume Eleven Conservation Science 2016 Western Australia Review and synthesis of knowledge of insular ecology, with emphasis on the islands of Western Australia IAN ABBOTT and ALLAN WILLS i TABLE OF CONTENTS Page ABSTRACT 1 INTRODUCTION 2 METHODS 17 Data sources 17 Personal knowledge 17 Assumptions 17 Nomenclatural conventions 17 PRELIMINARY 18 Concepts and definitions 18 Island nomenclature 18 Scope 20 INSULAR FEATURES AND THE ISLAND SYNDROME 20 Physical description 20 Biological description 23 Reduced species richness 23 Occurrence of endemic species or subspecies 23 Occurrence of unique ecosystems 27 Species characteristic of WA islands 27 Hyperabundance 30 Habitat changes 31 Behavioural changes 32 Morphological changes 33 Changes in niches 35 Genetic changes 35 CONCEPTUAL FRAMEWORK 36 Degree of exposure to wave action and salt spray 36 Normal exposure 36 Extreme exposure and tidal surge 40 Substrate 41 Topographic variation 42 Maximum elevation 43 Climate 44 Number and extent of vegetation and other types of habitat present 45 Degree of isolation from the nearest source area 49 History: Time since separation (or formation) 52 Planar area 54 Presence of breeding seals, seabirds, and turtles 59 Presence of Indigenous people 60 Activities of Europeans 63 Sampling completeness and comparability 81 Ecological interactions 83 Coups de foudres 94 LINKAGES BETWEEN THE 15 FACTORS 94 ii THE TRANSITION FROM MAINLAND TO ISLAND: KNOWNS; KNOWN UNKNOWNS; AND UNKNOWN UNKNOWNS 96 SPECIES TURNOVER 99 Landbird species 100 Seabird species 108 Waterbird