Secondary Sexual Characteristics in Codfishes ([[Gadidae]]) in Relation to Sound Production, Habitat Use, and Social Behaviour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Goh, Ee Von (2018) the Status of Fish in Malaysian Diets and Potential Barriers to Increasing Consumption of Farmed Species
THE STATUS OF FISH IN MALAYSIAN DIETS AND POTENTIAL BARRIERS TO INCREASING CONSUMPTION OF FARMED SPECIES Ee Von Goh Thesis submitted to the University of Nottingham Malaysia Campus for the degree of Doctor of Philosophy 2018 Abstract………………………………………………………………………………………………………………………………………….i Dedications and Acknowledgements……………………………………………………………………………………………..ii List of Tables…………………………………………………………………………………………………………………………………iii List of Figures…………………………………………………………………………………………………………………………………v List of Abbreviations.…………………………………………………………………………………………………………………….vi Chapter 1: Introduction 1.1 The Concept of Sustainable Diet for Human Health…………………………………………………………1 1.1.1 Indicators for Sustainable Consumption…………………………………………………………..3 1.2 The Importance of Fish in the Livelihood of Malaysian ……………………………………………………5 1.3 Fish Consumption Pattern in Malaysia………………………………............................................... 8 1.4 Fish Purchasing Behaviour of Malaysian…………………………………………………………………………. 9 1.5 Status of Malaysian Fishery Industry……………………………………………………………………………. 12 1.5.1 Marine Capture Fisheries……………………………………………………………………………… 12 1.5.2 Aquaculture……………………………………………………………………………………………………13 1.5.3 Post-Harvest Utilisation………………………………………………………………………………… 14 1.5.4 Assessing the Sustainability of Fishery Production……………………………………….. 15 1.5.5 The (Un)Sustainability of The Wild Fish Supply……………………………………………… 16 1.5.6 General Perceptions of Fish Farming and Farmed Fish……………………………………20 1.6 Stakeholders’ Roles in Fish Sustainability……………………………………………………………………… 23 1.7 Addressing -

Investigating the Trophic Ecology of Five Species of Gadiformes in the Celtic Sea Combining Stable Isotopes and Gut Contents Louise Day
Investigating the trophic ecology of five species of Gadiformes in the Celtic Sea combining stable isotopes and gut contents Louise Day To cite this version: Louise Day. Investigating the trophic ecology of five species of Gadiformes in the Celtic Sea combining stable isotopes and gut contents . Agronomy. 2017. dumas-01634570 HAL Id: dumas-01634570 https://dumas.ccsd.cnrs.fr/dumas-01634570 Submitted on 14 Nov 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AGROCAMPUS OUEST CFR Angers CFR Rennes Année universitaire : 2016 - 2017 Mémoire de Fin d'Études Spécialité : d’Ingénieur de l’Institut Supérieur des Sciences agronomiques, agroalimentaires, horticoles et du paysage Agronomie Spécialisation (et option éventuelle) : de Master de l’Institut Supérieur des Sciences agronomiques, agroalimentaires, horticoles et du paysage Sciences Halieutiques et Aquacoles – d'un autre établissement (étudiant arrivé en M2) Ressources et Ecosystèmes Aquatiques Investigating the trophic ecology of five species of Gadiformes in the Celtic Sea combining stable isotopes -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Atlantic Zone Based on Satellite Data, and (3) Egg and Larval Distributions of Cod and Haddock from Cape Hatteras to the Laurentian Channel
Fisheries and Oceans Pêches et Océans Science Sciences C S A S S C É S Canadian Stock Assessment Secretariat Secrétariat canadien pour l’évaluation des stocks Proceedings Series 2000/17 Série des compte rendus 2000/17 Final Report of the 2000 Annual Meeting of the Fisheries Oceanography Committee Including the Report of the Workshop on the Cod Recruitment Dilemma February 22-25, 2000 Northwest Atlantic Fisheries Center St. John’s, Newfoundland 1D.P. Swain, Chairperson (FOC) 2M. Castonguay, Chairperson (Cod Recruitment Workshop) 1Fisheries and Oceans Canada 343 Ave Université Ave Moncton, New-Brunswick E1C 9B6 2Fisheries and Oceans Canada Institut Maurice Lamontagne 850 Route de la Mer, C.P. 1000 G5H 3Z4 September 2000 ii Executive Summary of the 2000 FOC Annual Meeting The Fisheries Oceanography Committee (FOC) of the Department of Fisheries and Oceans (DFO) met in St. John’s, Newfoundland at the Northwest Atlantic Fisheries Center on 22-25 February 2000. The Committee reviewed environmental conditions in the Northwest Atlantic during 1999, convened a workshop on the Cod Recruitment Dilemma, reviewed additional papers on physical and biological oceanography and on changes in cod diets, and conducted its annual business meeting. 1. Physical Environment in 1999: Eight papers were reviewed on the meteorological and physical oceanographic conditions in 1999. Air temperature warmed relative to 1998 throughout most of the northwest Atlantic, reaching record high values in the Gulf of St. Lawrence, on the Scotian Shelf and over eastern Newfoundland. Sea ice coverage and duration were below average in most areas. Water temperatures from southern Labrador to the Grand Bank and off southern Newfoundland were generally above normal values.This was reflected in below-average volumes of the cold intermediate layer (CIL) and warmer- than-average bottom waters off Newfoundland, especially on the Grand Bank where bottom temperatures were 1-3°C above average. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Special Publication No. 6
SECTION C 435 C-l C-1 REMARKS ON EFFECT OF FOOD ANIMALS ON COD BEHAVIOUR By Sv. Aa. Horsted and Erik Smidt1 ABSTRACT In Greenland waters cod make long spawning and feeding migrations. The most important feeding ugrations take place in early summer, when cod follow the capelin into the fjords to the shore, lnd later in the summer, when cod follow the launce over the banks in the Davis Strait and in coastal iaters. Later on cod have been observed feeding quite near the shore in coastal areas, where the food consists of small capelin, Arctic squid and euphausiids. Cod may also be concentrated near lcebergs in summer and autumn. Finally. when cod have disappeared from the upper water layers be :ause of winter cooling, large numbers can be taken on the prawn grounds, where PandaZua borealis Ls the main food. COD PURSUING THE CAPELIN INTO THE FJORDS IN THE EARLY SUMMER In West Greenland large shoals of capelin (Mal lotus villosus) migrate into many 'of the fjords :or spawning, and in Southwest Greenland they are often pursued by cod. Both when the capelin ,efore spawning swim in shoals over the deeper parts of the fjords and coastal waters and when they ~ather near shore to spawn, one can follow the cod hunting them right up to the surface (Hansen, L949, p. 40), and investigations of cod stomachs show that they are full of capelin. During this Jeriod it is often difficult to catch cod with jig or long-line, as they pay no attention to the looks, even when baited with fresh capelin. -

MELANONIDAE Melanonus Zugmayeri Norman, 1930
click for previous page Gadiformes: Melanonidae 1001 MELANONIDAE Pelagic cods by T. Iwamoto, California Academy of Sciences, USA and D. M. Cohen, Bodega Bay, California, USA Melanonus zugmayeri Norman, 1930 En - Tropical pelagic cod. Diagnostic characters: Body slender, tapering to a narrow caudal peduncle. Head covered with free neuromasts aligned longitudinally into short ridges; pores of sensory lateralis system on head large, prominent; mouth large; teeth in 2 or 3 series in jaws, inner series laterally in lower jaw large, canine-like, widely spaced; teeth on vomer and palatines; no chin barbel. One long-based dorsal fin, high anteriorly, slightly notched at about sixth to tenth ray; anal fin long-based, rays finer than opposites of dorsal fin; caudal fin poorly developed, narrow, rounded to somewhat pointed; pectoral fin midlateral, below origin of dorsal fin; pelvic fin with 7 rays, origin anterior to pectoral base. Colour: overall blackish. Similar families occurring in the area Bathygadidae: no caudal fin; no teeth on roof of mouth; 2 separate dorsal fins, second ray of first dorsal fin a flexible spine, slightly to extremely prolonged. Gadidae: 2 or 3 separate dorsal fins; 1 or 2 anal fins; chin barbel present. 2nd ray is a 2 or 3 separate dorsal fins flexible 2 separate dorsal fins spine Bathygadidae chin Gadidae barbel Macrouridae: no caudal fin; no teeth on roof of mouth; scales usually covered with spinules. Moridae: 2 or 3 dorsal fins, 1or 2 anal fins, pelvic fins narrow with filamentous tips in some species; chin barbel developed in many; no enlarged, canine-like teeth in lower jaw, few or no teeth on vomer; swimbladder with an- terior projections that connect to rear of skull. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

And Saithe (Pollachius Virens) a Case Study Investigating the Juveniles’ Feeding Pattern and Identifying Valuable Nursery Habitats in the Icelandic Westfjords
Trophic vulnerability of 0-group Atlantic cod (Gadus morhua) and saithe (Pollachius virens) A case study investigating the juveniles’ feeding pattern and identifying valuable nursery habitats in the Icelandic Westfjords Anja Katrin Nickel Advisor: Dr. Guðbjörg Ásta Ólafsdóttir University of Akureyri Faculty of Business and Science University Centre of the Westfjords Master of Resource Management: Coastal and Marine Management Ísafjörður, August 2016 Supervisory Committee Advisor: Dr. Guðbjörg Ásta Ólafsdóttir University of Iceland Reader: Name, title Program Director: Dagný Arnarsdóttir, MSc. Anja Katrin Nickel Trophic vulnerability of 0-group Atlantic cod (Gadus morhua) and saithe (Pollachius virens): A case study investigating the juveniles’ feeding pattern and identifying valuable nursery habitats in the Icelandic Westfjords 45 ECTS thesis submitted in partial fulfilment of a Master of Resource Management degree in Coastal and Marine Management at the University Centre of the Westfjords, Suðurgata 12, 400 Ísafjörður, Iceland Degree accredited by the University of Akureyri, Faculty of Business and Science, Borgir, 600 Akureyri, Iceland Copyright © 2016 Anja Katrin Nickel All rights reserved Printing: Háskólaprent, Reykjavík, August 2016 Declaration I hereby confirm that I am the sole author of this thesis and it is a product of my own academic research. __________________________________________ Student‘s name Abstract Rapid environmental change due to anthropogenic impacts currently threaten marine ecosystems and increase the pressure on the vulnerable early life stages of many marine organisms. In this study I examine trophic vulnerability of 0-group Atlantic cod (Gadus morhua) and saithe (Pollachius virens) during late summer and fall. This period coincides with the Atlantic cod juvenile settlement from the pelagic to the benthic habitat in the northwest of Iceland. -

Stomach Data from Pelagic Research Survey Bring New Information in Cod Feeding Pattern in the South-Western Baltic Sea
Department of Aquatic Resources Stomach data from pelagic research survey bring new information in cod feeding pattern in the South-Western Baltic sea Federico Maioli Master thesis • 30 hec Lysekil 2019 Stomach data from pelagic research survey bring new information in cod feeding pattern in the South-Western Baltic sea Federico Maioli Supervisor: Michele Casini, Swedish University of Agricultural Sciences, Department of Aquatic Resources Assistant supervisor: Håkan Wennhage, Swedish University of Agricultural Sciences, Department of Aquatic Resources Examiner: Sara Königson, Swedish University of Agricultural Sciences, Department of Aquatic Resources Credits: 30 hec Level: Second cycle, A2E Course title: Indipendent project in biology Course code: EX0895 Place of publication: Lysekil Year of publication: 2019 Online publication: https://stud.epsilon.slu.se Keywords: Eastern Baltic Cod, diet, stomach data. Swedish University of Agricultural Sciences Faculty of Natural Resources and Agricultural Sciences Department of Aquatic Resources Abstract Pelagic and demersal cod (Gadus Morhua) specimens were collected respectively during a pelagic and a demersal survey in the Eastern Baltic sea in the fourth quarter of the years 2015-2017. Stomach contents were analysed and compared for the pur- pose of evaluating differences in the diet among pelagic and demersal specimens. Furthermore, generalized additive models (GAMs) were employed to investigate the daily fluctuations of stomach content weights of this predator. My results showed significant differences in the diet composition of demersal and pelagic cod mainly attributable to the higher weight share of sprat in the pelagic stomachs. Moreover, a remarkable diel variation in the stomach contents weights was present, indicating morning and evening peaks. The present study furnished novel insights into cod feed- ing pattern in the South-Western Baltic Sea. -
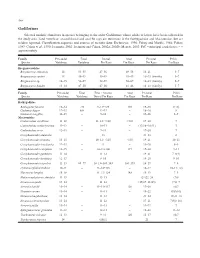
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Reducing the Conflict Between Cormorants and Fisheries on a Pan-European Scale
Reducing the conflict between Cormorants and fisheries on a pan-European scale REDCAFE Final Report Report of a Concerted Action funded by the European Union. Study contract no. Q5CA-2000-31387: Reducing the conflict between cormorants and fisheries on a pan-European scale. Edited by D N Carss Centre for Ecology & Hydrology Banchory, Hill of Brathens, Banchory Aberdeenshire, AB31 4BW, Scotland, UK. Natural Environment Research Council Centre for Ecology & Hydrology CEH Banchory Hill of Brathens Banchory Aberdeenshire AB31 4BY Scotland, UK. Fax: +44 1330 823303 Internet: [email protected] Web: www.banchory.ceh.ac.uk CEH Contract Number: C01749 Reducing the conflict between cormorants and fisheries on a pan-European scale Final Report Edited by: D N Carss Commissioned by: European Commission DG XIV Directorate-General for Fisheries Rue de la Loi 200 Bâtiment J II 99 6/11 B-1049 Brussels, Belgium Contract number: Q5CA-2000-31387 This report does not necessarily reflect the views of the European Commission and in no way anticipates any future opinion of the Commission. The contents of the report may not be reproduced unless the source of the material is indicated. This study has been carried out with the financial assistance of the European Commission. This report is drafted at the request of the commissioner indicated above and is his property. Nothing from this report may be reproduced and/or published by print, photoprint microfilm or any other means without the previous written consent from the commissioner of the study. Photographs on the cover (Top left, clockwise): Great cormorant © David Grémillet, coastal fisheries, Greece © Dave Carss, recreational fisherman © Trout & Salmon magazine, Danish pound net fishermen, © DC, Roach © Roger Phillips and Martyn Rix, REDCAFE participants, Waltham Abbey, November 2002, © Szymon Bzoma.