Thermodynamic Cycles • Look at Different Cycles That Approximate Real Processes • You Can Categorize These Processes Several Different Ways • Power Cycles Vs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constant Volume Combustion Cycle for IC Engines
Constant Volume Combustion Cycle for IC Engines Jovan Dorić Assistant This paper presents an analysis of the internal combustion engine cycle in University of Novi Sad Faculty of Technical Sciences cases when the new unconventional piston motion law is used. The main goal of the presented unconventional piston motion law is to make a Ivan Klinar realization of combustion during constant volume in an engine’s cylinder. Full Professor The obtained results are shown in PV (pressure-volume) diagrams of University of Novi Sad Faculty of Technical Sciences standard and new engine cycles. The emphasis is placed on the shortcomings of the real cycle in IC engines as well as policies that would Marko Dorić contribute to reducing these disadvantages. For this paper, volumetric PhD student efficiency, pressure and temperature curves for standard and new piston University of Novi Sad Faculty of Agriculture motion law have been calculated. Also, the results of improved efficiency and power are presented. Keywords: constant volume combustion, IC engine, kinematics. 1. INTRODUCTION of an internal combustion engine, whose combustion is so rapid that the piston does not move during the Internal combustion engine simulation modelling has combustion process, and thus combustion is assumed to long been established as an effective tool for studying take place at constant volume [6]. Although in engine performance and contributing to evaluation and theoretical terms heat addition takes place at constant new developments. Thermodynamic models of the real volume, in real engine cycle heat addition at constant engine cycle have served as effective tools for complete volume can not be performed. -

Steady State and Transient Efficiencies of A
STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Masters of Science Charles Van Horn August, 2006 STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE Charles Van Horn Thesis Approved: Accepted: Advisor Department Chair Dr. Scott Sawyer Dr. Celal Batur Faculty Reader Dean of the College Dr. Richard Gross Dr. George K. Haritos Faculty Reader Dean of the Graduate School Dr. Iqbal Husain Dr. George R. Newkome Date ii ABSTRACT The efficiencies of a four cylinder direct injection diesel engine have been investigated for the implementation in a hybrid electric vehicle (HEV). The engine was cycled through various operating points depending on the power and torque requirements for the HEV. The selected engine for the HEV is a 2005 Volkswagen 1.9L diesel engine. The 2005 Volkswagen 1.9L diesel engine was tested to develop the steady-state engine efficiencies and to evaluate the transient effects on these efficiencies. The peak torque and power curves were developed using a water brake dynamometer. Once these curves were obtained steady-state testing at various engine speeds and powers was conducted to determine engine efficiencies. Transient operation of the engine was also explored using partial throttle and variable throttle testing. The transient efficiency was compared to the steady-state efficiencies and showed a decrease from the steady- state values. -

Hybrid Electric Vehicles: a Review of Existing Configurations and Thermodynamic Cycles
Review Hybrid Electric Vehicles: A Review of Existing Configurations and Thermodynamic Cycles Rogelio León , Christian Montaleza , José Luis Maldonado , Marcos Tostado-Véliz * and Francisco Jurado Department of Electrical Engineering, University of Jaén, EPS Linares, 23700 Jaén, Spain; [email protected] (R.L.); [email protected] (C.M.); [email protected] (J.L.M.); [email protected] (F.J.) * Correspondence: [email protected]; Tel.: +34-953-648580 Abstract: The mobility industry has experienced a fast evolution towards electric-based transport in recent years. Recently, hybrid electric vehicles, which combine electric and conventional combustion systems, have become the most popular alternative by far. This is due to longer autonomy and more extended refueling networks in comparison with the recharging points system, which is still quite limited in some countries. This paper aims to conduct a literature review on thermodynamic models of heat engines used in hybrid electric vehicles and their respective configurations for series, parallel and mixed powertrain. It will discuss the most important models of thermal energy in combustion engines such as the Otto, Atkinson and Miller cycles which are widely used in commercial hybrid electric vehicle models. In short, this work aims at serving as an illustrative but descriptive document, which may be valuable for multiple research and academic purposes. Keywords: hybrid electric vehicle; ignition engines; thermodynamic models; autonomy; hybrid configuration series-parallel-mixed; hybridization; micro-hybrid; mild-hybrid; full-hybrid Citation: León, R.; Montaleza, C.; Maldonado, J.L.; Tostado-Véliz, M.; Jurado, F. Hybrid Electric Vehicles: A Review of Existing Configurations 1. Introduction and Thermodynamic Cycles. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Generation of Entropy in Spark Ignition Engines
Int. J. of Thermodynamics ISSN 1301-9724 Vol. 10 (No. 2), pp. 53-60, June 2007 Generation of Entropy in Spark Ignition Engines Bernardo Ribeiro, Jorge Martins*, António Nunes Universidade do Minho, Departamento de Engenharia Mecânica 4800-058 Guimarães PORTUGAL [email protected] Abstract Recent engine development has focused mainly on the improvement of engine efficiency and output emissions. The improvements in efficiency are being made by friction reduction, combustion improvement and thermodynamic cycle modification. New technologies such as Variable Valve Timing (VVT) or Variable Compression Ratio (VCR) are important for the latter. To assess the improvement capability of engine modifications, thermodynamic analysis of indicated cycles of the engines is made using the first and second laws of thermodynamics. The Entropy Generation Minimization (EGM) method proposes the identification of entropy generation sources and the reduction of the entropy generated by those sources as a method to improve the thermodynamic performance of heat engines and other devices. A computer model created and implemented in MATLAB Simulink was used to simulate the conventional Otto cycle and the various processes (combustion, free expansion during exhaust, heat transfer and fluid flow through valves and throttle) were evaluated in terms of the amount of the entropy generated. An Otto cycle, a Miller cycle (over-expanded cycle) and a Miller cycle with compression ratio adjustment are studied using the referred model in order to evaluate the amount of entropy generated in each cycle. All cycles are compared in terms of work produced per cycle. Keywords: IC engines, Miller cycle, entropy generation, over-expanded cycle 1. -

Measuring the Steady State Thermal Efficiency of a Heating System
Measuring the Steady State Thermal Efficiency of a Heating System. Last week you had a tour of “Colby Power and Light” and had a chance to see Gus Libby’s really cool, campus-scale, multi-million dollar calorimeter. This week we will use lab-scale calorimeters to measure the heat and carbon content of different fuels (methane, propane, butane, and gasoline). Our simple calorimeters were designed by Whitney King and Chuck Jones, Science Division Instrument Wizard, to simulate the highly efficient HTP Versa Flame fire-tube boiler. The lab has three parts: 1) Calibrate the calorimeter 2) Measure the heat content of different fuels 3) Compare the measured heat content to the calculated heat content 1) Calibrate the Calorimeter. You lab instructor will demonstrate the assembly of the laboratory- scale fire-tube calorimeter. The heat transferred from the burning fuel to the calorimeter can be determined from the mass of the object being heated, the heat capacity of the object and the change in temperature. � = ���� ∗ �� ∗ ∆� We will begin the experiment by adding a known amount of heat to our calorimeters from a coffee cup heater. The heater is a simple resistor that converts electrical energy into heat. The heater has a resistance of 47.5 ohms and is plugged into a 120 V outlet. Recalling your electricity basics, we know that V=IR where V is the voltage in Figure 1. HTP Versa Flame Fire Tube volts, I is the current in amps, and R is the Boiler. Brown Tubes carry the hot resistance in ohms. The power, P, consumed by combustion gasses inside of the the heater is volts times current, water tank where they condense releasing the maximum available 2 heat. -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

An Analytic Study of Applying Miller Cycle to Reduce Nox Emission from Petrol Engine Yaodong Wang, Lin Lin, Antony P
An analytic study of applying miller cycle to reduce nox emission from petrol engine Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, Yunxin He, Xiaodong Huang, Huilan Huang, Haiyan Wei, Shangping Li, et al. To cite this version: Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, et al.. An analytic study of applying miller cycle to reduce nox emission from petrol engine. Applied Thermal Engineering, Elsevier, 2007, 27 (11-12), pp.1779. 10.1016/j.applthermaleng.2007.01.013. hal-00498945 HAL Id: hal-00498945 https://hal.archives-ouvertes.fr/hal-00498945 Submitted on 9 Jul 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript An analytic study of applying miller cycle to reduce nox emission from petrol engine Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, Yunxin He, Xiaodong Huang, Huilan Huang, Haiyan Wei, Shangping Li, J Yang PII: S1359-4311(07)00031-2 DOI: 10.1016/j.applthermaleng.2007.01.013 Reference: ATE 2070 To appear in: Applied Thermal Engineering Received Date: 5 May 2006 Revised Date: 2 January 2007 Accepted Date: 14 January 2007 Please cite this article as: Y. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

Heat Cycles, Heat Engines, & Real Devices
Heat Cycles, Heat Engines, & Real Devices John Jechura – [email protected] Updated: January 4, 2015 Topics • Heat engines / heat cycles . Review of ideal‐gas efficiency equations . Efficiency upper limit –Carnot Cycle • Water as working fluid in Rankine Cycle . Role of rotating equipment inefficiency • Advanced heat cycles . Reheat & heat recycle • Organic Rankine Cycle • Real devices . Gas & steam turbines 2 Heat Engines / Heat Cycles • Carnot cycle . Most efficient heat cycle possible Hot Reservoir @ T H • Rankine cycle Q H . Usually uses water (steam) as working fluid W . Creates the majority of electric power used net throughout the world Q C . Can use any heat source, including solar thermal, Cold Sink @ T coal, biomass, & nuclear C • Otto cycle . Approximates the pressure & volume of the combustion chamber of a spark‐ignited engine WQQ • Diesel cycle net H C th QQ . Approximates the pressure & volume of the HH combustion chamber of the Diesel engine 3 Carnot Cycle • Most efficient heat cycle possible • Steps . Reversible isothermal expansion of gas at TH. Combination of heat absorbed from hot reservoir & work done on the surroundings. Reversible isentropic & adiabatic expansion of the gas to TC. No heat transferred & work done on the surroundings. Reversible isothermal compression of gas at TC. Combination of heat released to cold sink & work done on the gas by the surroundings. Reversible isentropic & adiabatic compression of the gas to TH. No heat transferred & work done on the gas by the surroundings. • Thermal efficiency QQHC TT HC T C th th 1 QTTHHH 4 Rankine/Brayton Cycle • Different application depending on working fluid . Rankine cycle to describe closed steam cycle. -

Review of the Fundamentals Thermal Sciences
Review of the Fundamentals Reading Problems Review Chapter 3 and property tables More specifically, look at: 3.2 ! 3.4, 3.6, 3.7, 8.4, 8.5, 8.6, 8.8 Thermal Sciences The thermal sciences involve the storage, transfer and conversion of energy. Thermodynamics HeatTransfer Conservationofmass Conduction Conservationofenergy Convection Secondlawofthermodynamics Radiation HeatTransfer Properties Conjugate Thermal Thermodynamics Systems Engineering FluidsMechanics FluidMechanics Fluidstatics Conservationofmomentum Mechanicalenergyequation Modeling Thermodynamics: the study of energy, energy transformations and its relation to matter. The anal- ysis of thermal systems is achieved through the application of the governing conservation equations, namely Conservation of Mass, Conservation of Energy (1st law of thermodynam- ics), the 2nd law of thermodynamics and the property relations. Heat Transfer: the study of energy in transit including the relationship between energy, matter, space and time. The three principal modes of heat transfer examined are conduction, con- vection and radiation, where all three modes are affected by the thermophysical properties, geometrical constraints and the temperatures associated with the heat sources and sinks used to drive heat transfer. Fluid Mechanics: the study of fluids at rest or in motion. While this course will not deal exten- sively with fluid mechanics we will be influenced by the governing equations for fluid flow, namely Conservation of Momentum and Conservation of Mass. 1 Examples of Energy Conversion windmills -
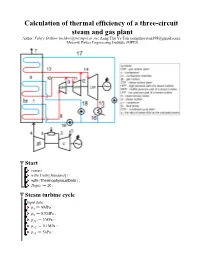
Calculation of Thermal Efficiency of a Three-Circuit Steam and Gas Plant
Calculation of thermal efficiency of a three-circuit steam and gas plant Author: Valery Ochkov ([email protected]), Aung Thu Ya Tun ([email protected]), Moscow Power Engineering Institute (MPEI) Start > > > > Steam turbine cycle Input data: > > > > > > > > > > > > > > Functions and procedures The specific enthalpy of the steam entering the turbine HPP: > (2.1) The specific enthalpy of steam at outlet of the turbine HPP: > (2.2) Specific work of HPP steam turbine: > (2.3) The temperature of the steam at the outlet of the HPP turbine: > (2.4) The specific enthalpy of steam at the point 10: > (2.5) > (2.6) The balance of the mixing streams at points 9 and 10: > (2.7) The temperature of steam at the inlet to the MPP of the turbine: > (2.8) > (2.8) > (2.9) > Specific enthalpy of water vapor at the inlet to the MPP of the turbine: > (2.10) Specific enthalpy of steam at the outlet of the turbine MPP: > (2.11) C : > (2.12) The temperature of the steam at the outlet of the MPP of the turbine: > (2.13) The specific enthalpy of steam at point 13: > (2.14) > (2.15) Balance when mixing streams at points 12 and 13: > (2.16) The temperature of steam at the inlet to the LPP of the turbine: > (2.17) > (2.18) The specific enthalpy of steam at outlet of the turbine LPP: > (2.19) Specific LPP operation of a steam turbine: > (2.8) > (2.20) The degree of dryness of the steam at the outlet of the turbine LPP: > (2.21) Condensate temperature: > (2.22) Specific enthalpy of condensate: > (2.23) The specific enthalpy of the feed water high pressure