Heat Cycles, Heat Engines, & Real Devices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constant Volume Combustion Cycle for IC Engines
Constant Volume Combustion Cycle for IC Engines Jovan Dorić Assistant This paper presents an analysis of the internal combustion engine cycle in University of Novi Sad Faculty of Technical Sciences cases when the new unconventional piston motion law is used. The main goal of the presented unconventional piston motion law is to make a Ivan Klinar realization of combustion during constant volume in an engine’s cylinder. Full Professor The obtained results are shown in PV (pressure-volume) diagrams of University of Novi Sad Faculty of Technical Sciences standard and new engine cycles. The emphasis is placed on the shortcomings of the real cycle in IC engines as well as policies that would Marko Dorić contribute to reducing these disadvantages. For this paper, volumetric PhD student efficiency, pressure and temperature curves for standard and new piston University of Novi Sad Faculty of Agriculture motion law have been calculated. Also, the results of improved efficiency and power are presented. Keywords: constant volume combustion, IC engine, kinematics. 1. INTRODUCTION of an internal combustion engine, whose combustion is so rapid that the piston does not move during the Internal combustion engine simulation modelling has combustion process, and thus combustion is assumed to long been established as an effective tool for studying take place at constant volume [6]. Although in engine performance and contributing to evaluation and theoretical terms heat addition takes place at constant new developments. Thermodynamic models of the real volume, in real engine cycle heat addition at constant engine cycle have served as effective tools for complete volume can not be performed. -

Hybrid Electric Vehicles: a Review of Existing Configurations and Thermodynamic Cycles
Review Hybrid Electric Vehicles: A Review of Existing Configurations and Thermodynamic Cycles Rogelio León , Christian Montaleza , José Luis Maldonado , Marcos Tostado-Véliz * and Francisco Jurado Department of Electrical Engineering, University of Jaén, EPS Linares, 23700 Jaén, Spain; [email protected] (R.L.); [email protected] (C.M.); [email protected] (J.L.M.); [email protected] (F.J.) * Correspondence: [email protected]; Tel.: +34-953-648580 Abstract: The mobility industry has experienced a fast evolution towards electric-based transport in recent years. Recently, hybrid electric vehicles, which combine electric and conventional combustion systems, have become the most popular alternative by far. This is due to longer autonomy and more extended refueling networks in comparison with the recharging points system, which is still quite limited in some countries. This paper aims to conduct a literature review on thermodynamic models of heat engines used in hybrid electric vehicles and their respective configurations for series, parallel and mixed powertrain. It will discuss the most important models of thermal energy in combustion engines such as the Otto, Atkinson and Miller cycles which are widely used in commercial hybrid electric vehicle models. In short, this work aims at serving as an illustrative but descriptive document, which may be valuable for multiple research and academic purposes. Keywords: hybrid electric vehicle; ignition engines; thermodynamic models; autonomy; hybrid configuration series-parallel-mixed; hybridization; micro-hybrid; mild-hybrid; full-hybrid Citation: León, R.; Montaleza, C.; Maldonado, J.L.; Tostado-Véliz, M.; Jurado, F. Hybrid Electric Vehicles: A Review of Existing Configurations 1. Introduction and Thermodynamic Cycles. -

Generation of Entropy in Spark Ignition Engines
Int. J. of Thermodynamics ISSN 1301-9724 Vol. 10 (No. 2), pp. 53-60, June 2007 Generation of Entropy in Spark Ignition Engines Bernardo Ribeiro, Jorge Martins*, António Nunes Universidade do Minho, Departamento de Engenharia Mecânica 4800-058 Guimarães PORTUGAL [email protected] Abstract Recent engine development has focused mainly on the improvement of engine efficiency and output emissions. The improvements in efficiency are being made by friction reduction, combustion improvement and thermodynamic cycle modification. New technologies such as Variable Valve Timing (VVT) or Variable Compression Ratio (VCR) are important for the latter. To assess the improvement capability of engine modifications, thermodynamic analysis of indicated cycles of the engines is made using the first and second laws of thermodynamics. The Entropy Generation Minimization (EGM) method proposes the identification of entropy generation sources and the reduction of the entropy generated by those sources as a method to improve the thermodynamic performance of heat engines and other devices. A computer model created and implemented in MATLAB Simulink was used to simulate the conventional Otto cycle and the various processes (combustion, free expansion during exhaust, heat transfer and fluid flow through valves and throttle) were evaluated in terms of the amount of the entropy generated. An Otto cycle, a Miller cycle (over-expanded cycle) and a Miller cycle with compression ratio adjustment are studied using the referred model in order to evaluate the amount of entropy generated in each cycle. All cycles are compared in terms of work produced per cycle. Keywords: IC engines, Miller cycle, entropy generation, over-expanded cycle 1. -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

An Analytic Study of Applying Miller Cycle to Reduce Nox Emission from Petrol Engine Yaodong Wang, Lin Lin, Antony P
An analytic study of applying miller cycle to reduce nox emission from petrol engine Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, Yunxin He, Xiaodong Huang, Huilan Huang, Haiyan Wei, Shangping Li, et al. To cite this version: Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, et al.. An analytic study of applying miller cycle to reduce nox emission from petrol engine. Applied Thermal Engineering, Elsevier, 2007, 27 (11-12), pp.1779. 10.1016/j.applthermaleng.2007.01.013. hal-00498945 HAL Id: hal-00498945 https://hal.archives-ouvertes.fr/hal-00498945 Submitted on 9 Jul 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript An analytic study of applying miller cycle to reduce nox emission from petrol engine Yaodong Wang, Lin Lin, Antony P. Roskilly, Shengchuo Zeng, Jincheng Huang, Yunxin He, Xiaodong Huang, Huilan Huang, Haiyan Wei, Shangping Li, J Yang PII: S1359-4311(07)00031-2 DOI: 10.1016/j.applthermaleng.2007.01.013 Reference: ATE 2070 To appear in: Applied Thermal Engineering Received Date: 5 May 2006 Revised Date: 2 January 2007 Accepted Date: 14 January 2007 Please cite this article as: Y. -

3.5 the Internal Combustion Engine (Otto Cycle)
Thermodynamics and Propulsion Next: 3.6 Diesel Cycle Up: 3. The First Law Previous: 3.4 Refrigerators and Heat Contents Index Subsections 3.5.1 Efficiency of an ideal Otto cycle 3.5.2 Engine work, rate of work per unit enthalpy flux 3.5 The Internal combustion engine (Otto Cycle) [VW, S & B: 9.13] The Otto cycle is a set of processes used by spark ignition internal combustion engines (2-stroke or 4- stroke cycles). These engines a) ingest a mixture of fuel and air, b) compress it, c) cause it to react, thus effectively adding heat through converting chemical energy into thermal energy, d) expand the combustion products, and then e) eject the combustion products and replace them with a new charge of fuel and air. The different processes are shown in Figure 3.8: 1. Intake stroke, gasoline vapor and air drawn into engine ( ). 2. Compression stroke, , increase ( ). 3. Combustion (spark), short time, essentially constant volume ( ). Model: heat absorbed from a series of reservoirs at temperatures to . 4. Power stroke: expansion ( ). 5. Valve exhaust: valve opens, gas escapes. 6. ( ) Model: rejection of heat to series of reservoirs at temperatures to . 7. Exhaust stroke, piston pushes remaining combustion products out of chamber ( ). We model the processes as all acting on a fixed mass of air contained in a piston-cylinder arrangement, as shown in Figure 3.10. Figure 3.8: The ideal Otto cycle Figure 3.9: Sketch of an actual Otto cycle Figure 3.10: Piston and valves in a four-stroke internal combustion engine The actual cycle does not have the sharp transitions between the different processes that the ideal cycle has, and might be as sketched in Figure 3.9. -

Performance Analysis of a Diesel Cycle Under the Restriction of Maximum Cycle Temperature with Considerations of Heat Loss, Friction, and Variable Specific Heats S.S
Vol. 120 (2011) ACTA PHYSICA POLONICA A No. 6 Performance Analysis of a Diesel Cycle under the Restriction of Maximum Cycle Temperature with Considerations of Heat Loss, Friction, and Variable Specific Heats S.S. Houa;¤ and J.C. Linb aDepartment of Mechanical Engineering, Kun Shan University, Tainan City 71003, Taiwan, ROC bDepartment of General Education, Transworld University, Touliu City, Yunlin County 640, Taiwan, ROC (Received November 11, 2010) The objective of this study is to examine the influences of heat loss characterized by a percentage of fuel’s energy, friction and variable specific heats of working fluid on the performance of an air standard Diesel cycle with the restriction of maximum cycle temperature. A more realistic and precise relationship between the fuel’s chemical energy and the heat leakage that is constituted on a pair of inequalities is derived through the resulting temperature. The variations in power output and thermal efficiency with compression ratio, and the relations between the power output and the thermal efficiency of the cycle are presented. The results show that the power output as well as the efficiency where maximum power output occurs will increase with the increase of maximum cycle temperature. The temperature-dependent specific heats of working fluid have a significant influence on the performance. The power output and the working range of the cycle increase while the efficiency decreases with increasing specific heats of working fluid. The friction loss has a negative effect on the performance. Therefore, the power output and efficiency of the cycle decrease with increasing friction loss. It is noteworthy that the effects of heat loss characterized by a percentage of fuel’s energy, friction and variable specific heats of working fluid on the performance of a Diesel-cycle engine are significant and should be considered in practice cycle analysis. -

Air Standard Assumptions Some Definitions for Reciprocation Engines
Air Standard Assumptions In power engines, energy is provided by burning fuel within the system boundaries, i.e., internal combustion engines. The following assumptions are commonly known as the air- standard assumptions: 1- The working fluid is air, which continuously circulates in a closed loop (cycle). Air is considered as ideal gas. 2- All the processes in (ideal) power cycles are internally reversible. 3- Combustion process is modeled by a heat-addition process from an external source. 4- The exhaust process is modeled by a heat-rejection process that restores the working fluid (air) at its initial state. Assuming constant specific heats, (@25°C) for air, is called cold-air-standard assumption. Some Definitions for Reciprocation Engines: The reciprocation engine is one the most common machines that is being used in a wide variety of applications from automobiles to aircrafts to ships, etc. Intake Exhaust valve valve TDC Bore Stroke BDC Fig. 3-1: Reciprocation engine. Top dead center (TDC): The position of the piston when it forms the smallest volume in the cylinder. Bottom dead center (BDC): The position of the piston when it forms the largest volume in the cylinder. Stroke: The largest distance that piston travels in one direction. Bore: The diameter of the piston. M. Bahrami ENSC 461 (S 11) IC Engines 1 Clearance volume: The minimum volume formed in the cylinder when the piston is at TDC. Displacement volume: The volume displaced by the piston as it moves between the TDC and BDC. Compression ratio: The ratio of maximum to minimum (clearance) volumes in the cylinder: V V r max BDC Vmin VTDC Mean effective pressure (MEP): A fictitious (constant throughout the cycle) pressure that if acted on the piston will produce the work. -

Performance Study for Miller Cycle Natural Gas Engine Based on GT-Power
Journal of Clean Energy Technologies, Vol. 3, No. 5, September 2015 Performance Study for Miller Cycle Natural Gas Engine Based on GT-Power Songsong Song and Hongguang Zhang [4]. Abstract—In order to improve the thermal efficiency of However, few attentions have been paid to the working natural gas engine and reduce the NOx emission from engine, process of the natural gas engine. In this paper, GT-Power this paper presented the technical route which applied Miller software was used to simulate the turbocharged natural gas cycle to turbocharged lean-burn natural gas engine. GT-Power engine. BSFC and NO emission were analyzed for two software was used to model the turbocharged natural gas x engine, and simulation research based on Miller cycle was kinds of engine working processes based on Otto cycle and performed according to various engine loads in the engine Miller cycle respectively. speed range of 2000-5000rpm. The simulation results showed that, under part load (50% of the engine full load) operating conditions of the turbocharged lean-burn natural gas engine, II. METHODOLOGY compared with Otto cycle, Miller cycle can reduce the brake specific fuel consumption (BSFC) and NOx emission. Under In order to improve the thermal efficiency of the engine, high load and low speed as well as moderate load and high Ralph Miller proposed Miller cycle in 1947 [5], which is a speed operating conditions, Miller cycle can effectively reduce kind of over-expanded cycle, as shown in Fig. 1. the BSFC. Under high load and low speed or high speed operating conditions, the NOx emission can be reduced apparently. -

Power Plant 3
Centre of Excellence in Renewable Energy Education and Research, New Campus, University of Lucknow, Lucknow B Voc. Renewable Energy Technology Semester II First Year Module RET-203: Power Plant Engineering Unit-2 (Air Standard cycle and Diesel Electric Power Plant) Contents: Internal Combustion Engine and External Combustion Engine: Otto Cycle, Diesel Cycle, Dual Cycle, Efficiency and Indicator Diagram. Diesel Electric Power Plant: Working Principle, Layout, Performance and Thermal Efficiency, Combined Cycle Power Plant, Layout, Efficiency. Internal Combustion Engine and External Combustion Engine In an external combustion engine, the fuel isn't burned inside the engine. With an internal combustion engine, the combustion chamber lies right in the middle of the engine. An Internal combustion engines rely on the explosive power of the fuel within the engine to produce work. In internal combustion engines, the explosion forcefully pushes pistons or expels hot high-pressure gas out of the engine at great speeds. Both moving pistons and ejected high- speed gas have the ability to do work. In external combustion engines, combustion heats a fluid which, in turn, does all the work. Example: Atkinson, Brayton/Joule, Diesel, Otto, Gas Generator etc. An external combustion engine uses a working fluid, either a liquid or a gas or both, that is heated by a fuel burned outside the engine. The external combustion chamber is filled with a fuel and air mixture that is ignited to produce a large amount of heat. This heat is then used to heat the internal working fluid either through the engine wall or a heat exchanger. The fluid expands when heated, acting on the mechanism of the engine, thus producing motion and usable work. -

Efficiency at Maximum Power of the Low-Dissipation Hybrid
energies Article Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle David Diskin 1 and Leonid Tartakovsky 1,2,* 1 Faculty of Mechanical Engineering, Technion—Israel Institute of Technology, Haifa 3200003, Israel; [email protected] 2 Grand Technion Energy Program, Technion—Israel Institute of Technology, Haifa 3200003, Israel * Correspondence: [email protected] Received: 24 June 2020; Accepted: 24 July 2020; Published: 1 August 2020 Abstract: A novel analytical method was developed for analysis of efficiency at maximum power of a hybrid cycle combining electrochemical and Otto engines. The analysis is based on the low-dissipation model, which relates energy dissipation with energy transfer rate. Efficiency at maximum power of a hybrid engine operating between two reservoirs of chemical potentials is evaluated. The engine is composed of an electrochemical device that transforms chemical potential to electrical work of an Otto engine that uses the heat generated in the electrochemical device and its exhaust effluent for mechanical work production. The results show that efficiency at maximum power of the hybrid cycle is identical to the efficiency at maximum power of an electrochemical engine alone; however, the power is the product of the electrochemical engine power and the compression ratio of the Otto engine. Partial mass transition by the electrochemical device from the high to the low chemical potential is also examined. In the latter case, heat is generated both in the electrochemical device and the Otto engine, and the efficiency at maximum power is a function of the compression ratio. An analysis performed using the developed method shows, for the first time, that, in terms of a maximal power, at some conditions, Otto cycle can provide better performance that the hybrid cycle. -
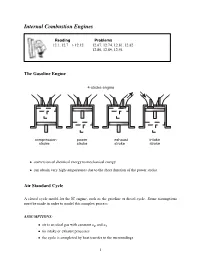
Internal Combustion Engines
Internal Combustion Engines Reading Problems 12.1, 12.7 ! 12.12 12.67, 12.74, 12.81, 12.82 12.86, 12.89, 12.94 The Gasoline Engine • conversion of chemical energy to mechanical energy • can obtain very high temperatures due to the short duration of the power stroke Air Standard Cycle A closed cycle model for the IC engine, such as the gasoline or diesel cycle. Some assumptions must be made in order to model this complex process. ASSUMPTIONS: • air is an ideal gas with constant cp and cv • no intake or exhaust processes • the cycle is completed by heat transfer to the surroundings 1 • the internal combustion process is replaced by a heat transfer process from a TER • all internal processes are reversible • heat addition occurs instantaneously while the piston is at TDC Definitions Mean Effective Pressure (MEP): The theoretical constant pressure that, if it acted on the piston during the power stroke would produce the same net work as actually developed in one complete cycle. net work for one cycle W MEP = = net displacement volume VBDC − VT DC The mean effective pressure is an index that relates the work output of the engine to it size (displacement volume). Otto Cycle • the theoretical model for the gasoline engine • consists of four internally reversible processes • heat is transferred to the working fluid at constant volume Otto Cycle Efficiency W Q − Q Q Q η = net = H L = 1 − L = 1 − 4−1 QH QH QH Q2−3 QH = mcv(T3 − T2)(intake) QL = mcv(T4 − T1)(exhaust) Therefore ! T4 ! − 1 (T4 − T1) T1 T1 η = 1 − = 1 − ! (T − T ) T T3 3 2 2 − 1 T2 2 Since