Air Standard Cycles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constant Volume Combustion Cycle for IC Engines
Constant Volume Combustion Cycle for IC Engines Jovan Dorić Assistant This paper presents an analysis of the internal combustion engine cycle in University of Novi Sad Faculty of Technical Sciences cases when the new unconventional piston motion law is used. The main goal of the presented unconventional piston motion law is to make a Ivan Klinar realization of combustion during constant volume in an engine’s cylinder. Full Professor The obtained results are shown in PV (pressure-volume) diagrams of University of Novi Sad Faculty of Technical Sciences standard and new engine cycles. The emphasis is placed on the shortcomings of the real cycle in IC engines as well as policies that would Marko Dorić contribute to reducing these disadvantages. For this paper, volumetric PhD student efficiency, pressure and temperature curves for standard and new piston University of Novi Sad Faculty of Agriculture motion law have been calculated. Also, the results of improved efficiency and power are presented. Keywords: constant volume combustion, IC engine, kinematics. 1. INTRODUCTION of an internal combustion engine, whose combustion is so rapid that the piston does not move during the Internal combustion engine simulation modelling has combustion process, and thus combustion is assumed to long been established as an effective tool for studying take place at constant volume [6]. Although in engine performance and contributing to evaluation and theoretical terms heat addition takes place at constant new developments. Thermodynamic models of the real volume, in real engine cycle heat addition at constant engine cycle have served as effective tools for complete volume can not be performed. -

Steady State and Transient Efficiencies of A
STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Masters of Science Charles Van Horn August, 2006 STEADY STATE AND TRANSIENT EFFICIENCIES OF A FOUR CYLINDER DIRECT INJECTION DIESEL ENGINE FOR IMPLEMENTATION IN A HYBRID ELECTRIC VEHICLE Charles Van Horn Thesis Approved: Accepted: Advisor Department Chair Dr. Scott Sawyer Dr. Celal Batur Faculty Reader Dean of the College Dr. Richard Gross Dr. George K. Haritos Faculty Reader Dean of the Graduate School Dr. Iqbal Husain Dr. George R. Newkome Date ii ABSTRACT The efficiencies of a four cylinder direct injection diesel engine have been investigated for the implementation in a hybrid electric vehicle (HEV). The engine was cycled through various operating points depending on the power and torque requirements for the HEV. The selected engine for the HEV is a 2005 Volkswagen 1.9L diesel engine. The 2005 Volkswagen 1.9L diesel engine was tested to develop the steady-state engine efficiencies and to evaluate the transient effects on these efficiencies. The peak torque and power curves were developed using a water brake dynamometer. Once these curves were obtained steady-state testing at various engine speeds and powers was conducted to determine engine efficiencies. Transient operation of the engine was also explored using partial throttle and variable throttle testing. The transient efficiency was compared to the steady-state efficiencies and showed a decrease from the steady- state values. -

Benchmarking a 2018 Toyota Camry 2.5L Atkinson Cycle Engine With
Downloaded from SAE International by John Kargul, Thursday, April 04, 2019 2019-01-0249 Published 02 Apr 2019 Benchmarking a 2018 Toyota Camry 2.5-Liter INTERNATIONAL. Atkinson Cycle Engine with Cooled-EGR John Kargul, Mark Stuhldreher, Daniel Barba, Charles Schenk, Stanislav Bohac, Joseph McDonald, and Paul Dekraker US Environmental Protection Agency Josh Alden Southwest Research Institute Citation: Kargul, J., Stuhldreher, M., Barba, D., Schenk, C. et al., “Benchmarking a 2018 Toyota Camry 2.5-Liter Atkinson Cycle Engine with Cooled-EGR,” SAE Technical Paper 2019-01-0249, 2019, doi:10.4271/2019-01-0249. Abstract idle, low, medium, and high load engine operation. Motoring s part of the U.S. Environmental Protection Agency’s torque, wide open throttle (WOT) torque and fuel consumption (EPA’s) continuing assessment of advanced light-duty are measured during transient operation using both EPA Tier Aautomotive technologies in support of regulatory and 2 and Tier 3 test fuels. Te design and performance of this 2018 compliance programs, a 2018 Toyota Camry A25A-FKS 2.5-liter engine is described and compared to Toyota’s published 4-cylinder, 2.5-liter, naturally aspirated, Atkinson Cycle engine data and to EPA’s previous projections of the efciency of an with cooled exhaust gas recirculation (cEGR) was bench- Atkinson Cycle engine with cEGR. The Brake Thermal marked. Te engine was tested on an engine dynamometer Efciency (BTE) map for the Toyota A25A-FKS engine shows with and without its 8-speed automatic transmission, and with a peak efciency near 40 percent, which is the highest value of the engine wiring harness tethered to a complete vehicle parked any publicly available map for a non-hybrid production gasoline outside of the test cell. -
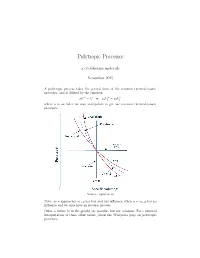
Polytropic Processes
Polytropic Processes a.cyclohexane.molecule November 2015 A polytropic process takes the general form of the common thermodynamic processes, and is defined by the equation n n n pV = C or p1V1 = p2V2 where n is an index we may manipulate to get our common thermodynamic processes. Source: nptel.ac.in Note: as n approaches 1, p has less and less influence; when n = 1, p has no influence and we thus have an isobaric process. Other n values (x in the graph) are possible, but not common. For a physical interpretation of these other values, check the Wikipedia page on polytropic processes. Because polytropic processes are general representations of thermodynamic pro- cesses, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes. We proceed to find the work and heat associated with a polytropic process. We start with the definition of work: Z V2 w = − p dV V1 The polytropic equation pV n = C allows us to manipulate our formula for work in the following manner, as long as n 6= 1 V V Z 2 C Z 2 C V C V w = − dV = −C V −n dV = − V 1−n 2 = V 1−n 2 n V1 V1 V1 V V1 1 − n n − 1 Evaluating this expression, C CV 1−n − CV 1−n w = V 1−n − V 1−n = 2 1 n − 1 2 1 n − 1 n n We let the first C be p2V2 and the second C be p1V1 , such that the exponents cancel to obtain p V nV 1−n − p V nV 1−n p V − p V R(T − T ) w = 2 2 2 1 1 1 = 2 2 1 1 = 2 1 n − 1 n − 1 n − 1 where the last equality follows from the ideal gas law pV = RT and our deriva- tion for the work of a polytropic process is complete. -

Hybrid Electric Vehicles: a Review of Existing Configurations and Thermodynamic Cycles
Review Hybrid Electric Vehicles: A Review of Existing Configurations and Thermodynamic Cycles Rogelio León , Christian Montaleza , José Luis Maldonado , Marcos Tostado-Véliz * and Francisco Jurado Department of Electrical Engineering, University of Jaén, EPS Linares, 23700 Jaén, Spain; [email protected] (R.L.); [email protected] (C.M.); [email protected] (J.L.M.); [email protected] (F.J.) * Correspondence: [email protected]; Tel.: +34-953-648580 Abstract: The mobility industry has experienced a fast evolution towards electric-based transport in recent years. Recently, hybrid electric vehicles, which combine electric and conventional combustion systems, have become the most popular alternative by far. This is due to longer autonomy and more extended refueling networks in comparison with the recharging points system, which is still quite limited in some countries. This paper aims to conduct a literature review on thermodynamic models of heat engines used in hybrid electric vehicles and their respective configurations for series, parallel and mixed powertrain. It will discuss the most important models of thermal energy in combustion engines such as the Otto, Atkinson and Miller cycles which are widely used in commercial hybrid electric vehicle models. In short, this work aims at serving as an illustrative but descriptive document, which may be valuable for multiple research and academic purposes. Keywords: hybrid electric vehicle; ignition engines; thermodynamic models; autonomy; hybrid configuration series-parallel-mixed; hybridization; micro-hybrid; mild-hybrid; full-hybrid Citation: León, R.; Montaleza, C.; Maldonado, J.L.; Tostado-Véliz, M.; Jurado, F. Hybrid Electric Vehicles: A Review of Existing Configurations 1. Introduction and Thermodynamic Cycles. -

MECH 230 – Thermodynamics 1 Final Workbook Solutions
MECH 230 – Thermodynamics 1 Final Workbook Solutions CREATED BY JUSTIN BONAL MECH 230 Final Exam Workbook Contents 1.0 General Knowledge ........................................................................................................................... 1 1.1 Unit Analysis .................................................................................................................................. 1 1.2 Pressure ......................................................................................................................................... 1 2.0 Energies ............................................................................................................................................ 2 2.1 Work .............................................................................................................................................. 2 2.2 Internal Energy .............................................................................................................................. 3 2.3 Heat ............................................................................................................................................... 3 2.4 First Law of Thermodynamics ....................................................................................................... 3 3.0 Ideal gas ............................................................................................................................................ 4 3.1 Universal Gas Constant ................................................................................................................. -

3. Thermodynamics Thermodynamics
Design for Manufacture 3. Thermodynamics Thermodynamics • Thermodynamics – study of heat related matter in motion. • Ma jor deve lopments d uri ng 1650 -1850 • Engineering thermodynamics mainly concerned with work produc ing or utili si ng machi nes such as - Engines, - TbiTurbines and - Compressors together w ith th e worki ng sub stances used i n th e machi nes. • Working substance – fluids: capable of deformation, energy transfer • Air an d s team are common wor king sub st ance Design and Manufacture Pressure F P = A Force per unit area. Unit: Pascal (Pa) N / m 2 1 Bar =105 Pa 1 standard atmosphere = 1.01325 Bar 1 Bar =14.504 Psi (pound force / square inch, 1N= 0.2248 pound force) 1 Psi= 6894.76 Pa Design and Manufacture Phase Nature of substance. Matter can exists in three phases: solid, liquid and gas Cycle If a substance undergoes a series of processes and return to its original state, then it is said to have been taken through a cycle. Design for Manufacture Process A substance is undergone a process if the state is changed by operation carried out on it Isothermal process - Constant temperature process Isobaric process - Constant pressure process Isometric process or isochoric process - Constant volume process Adiabatic Process No heat is transferred, if a process happens so quickly that there is no time to transfer heat, or the system is very well insulated from its surroundings. Polytropic process Occurs with an interchange of both heat and work between the system and its surroundings E.g. Nonadiabatic expansion or compression -

RECIPROCATING ENGINES Franck Nicolleau
RECIPROCATING ENGINES Franck Nicolleau To cite this version: Franck Nicolleau. RECIPROCATING ENGINES. Master. RECIPROCATING ENGINES, Sheffield, United Kingdom. 2010, pp.189. cel-01548212 HAL Id: cel-01548212 https://hal.archives-ouvertes.fr/cel-01548212 Submitted on 27 Jun 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Mechanical Engineering - 14 May 2010 -1- UNIVERSITY OF SHEFFIELD Department of Mechanical Engineering Mappin street, Sheffield, S1 3JD, England RECIPROCATING ENGINES Autumn Semester 2010 MEC403 - MEng, semester 7 - MEC6403 - MSc(Res) Dr. F. C. G. A. Nicolleau MD54 Telephone: +44 (0)114 22 27700. Direct Line: +44 (0)114 22 27867 Fax: +44 (0)114 22 27890 email: F.Nicolleau@sheffield.ac.uk http://www.shef.ac.uk/mecheng/mecheng cms/staff/fcgan/ MEng 4th year Course Tutor : Pr N. Qin European and Year Abroad Tutor : C. Pinna MSc(Res) and MPhil Course Director : F. C. G. A. Nicolleau c 2010 F C G A Nicolleau, The University of Sheffield -2- Combustion engines Table of content -3- Table of content Table of content 3 Nomenclature 9 Introduction 13 Acknowledgement 16 I - Introduction and Fundamentals of combustion 17 1 Introduction to combustion engines 19 1.1 Pistonengines.................................. -
Efficiency of Atkinson Engine at Maximum Power Density Using Temperature Dependent Specific Heats
Volume 2, Number 2, Jun. 2008 ISSN 1995-6665 JJMIE Pages 71 - 75 Jordan Journal of Mechanical and Industrial Engineering Efficiency of Atkinson Engine at Maximum Power Density using Temperature Dependent Specific Heats A. Al-Sarkhi, B. Akash *, E. Abu-Nada and I. Al-Hinti Department of Mechanical Engineering, Hashemite University, Zarqa, 13115, Jordan Abstract Thermodynamic analysis of an ideal air-standard Atkinson cycle with temperature dependant specific heat is presented in this paper. The paper outlines the effect of maximizing power density on the performance of the cycle efficiency. The power density is defined as the ratio of the power output to the maximum cycle specific volume. It showed significant effect on the performance of the cycle over the constant specific heat model. The results obtained from this work can be helpful in the thermodynamic modeling and in the evaluation of real Atkinson engines over other engines. © 2008 Jordan Journal of Mechanical and Industrial Engineering. All rights reserved Keywords: Atkinson engine; power density; temperature dependant specific heat; specific heats of air (the working fluid) were used. The maximization of the power density is defined as the ratio 1. Introduction of the maximum power to the maximum specific volume in the cycle. It takes into consideration the engine size The Atkinson cycle (the complete expansion cycle) is instead of just maximizing its power output. The inclusion named after its inventor James Atkinson in 1882 [1]. As of the engine size in the calculation of its performance is a shown in Fig. 1, the cycle involves isentropic compression very important factor from an economical point of view. -

Generation of Entropy in Spark Ignition Engines
Int. J. of Thermodynamics ISSN 1301-9724 Vol. 10 (No. 2), pp. 53-60, June 2007 Generation of Entropy in Spark Ignition Engines Bernardo Ribeiro, Jorge Martins*, António Nunes Universidade do Minho, Departamento de Engenharia Mecânica 4800-058 Guimarães PORTUGAL [email protected] Abstract Recent engine development has focused mainly on the improvement of engine efficiency and output emissions. The improvements in efficiency are being made by friction reduction, combustion improvement and thermodynamic cycle modification. New technologies such as Variable Valve Timing (VVT) or Variable Compression Ratio (VCR) are important for the latter. To assess the improvement capability of engine modifications, thermodynamic analysis of indicated cycles of the engines is made using the first and second laws of thermodynamics. The Entropy Generation Minimization (EGM) method proposes the identification of entropy generation sources and the reduction of the entropy generated by those sources as a method to improve the thermodynamic performance of heat engines and other devices. A computer model created and implemented in MATLAB Simulink was used to simulate the conventional Otto cycle and the various processes (combustion, free expansion during exhaust, heat transfer and fluid flow through valves and throttle) were evaluated in terms of the amount of the entropy generated. An Otto cycle, a Miller cycle (over-expanded cycle) and a Miller cycle with compression ratio adjustment are studied using the referred model in order to evaluate the amount of entropy generated in each cycle. All cycles are compared in terms of work produced per cycle. Keywords: IC engines, Miller cycle, entropy generation, over-expanded cycle 1. -

Performance Analysis of an Atkinson Cycle Engine Under Effective Power
Vol. 132 (2017) ACTA PHYSICA POLONICA A No. 4 Performance Analysis of an Atkinson Cycle Engine under Effective Power and Effective Power Density Conditions G. Gonca∗ Yildiz Technical University, Naval Arch. and Marine Eng. Depart., Besiktas, Istanbul, Turkey (Received November 16, 2016; in final form May 20, 2017) This study presents performance optimization of an Atkinson cycle engine using criteria named as effective power and effective power density conditions. The effects of design and operating parameters such as compression ratio, cycle pressure ratio, cycle temperature ratio, equivalence ratio, bore/stroke ratio, inlet temperature, inlet pressure, engine speed, friction coefficient, mean piston speed and stroke length, on the performance characteristics have been examined. Moreover, the energy losses have been determined as fuel energy and they have been classified as incomplete combustion losses, friction losses, heat transfer losses, and exhaust output losses. Realistic values of specific heats have been used depending on temperature of working fluid. The results of the study can be assessed as an engineering tool by the Atkinson cycle engine designers. DOI: 10.12693/APhysPolA.132.1306 PACS/topics: gasoline engine, Atkinson cycle, engine performance, power density, finite-time thermodynamics 1. Introduction on artificial neural network technique. Gahruei et al. [28] compared the performances of dual Diesel cycle and dual In the recent years, the researchers carried out several AC based on the FTT by considering irreversible energy studies [1–21] on the Miller cycle (MC) engine to dimin- losses. It was observed from the results that the perfor- ish NOx emissions by reducing the maximum combus- mance of dual AC are higher than that of dual Diesel tion temperatures of the engines. -

Engineering Fundamentals of the Internal Combustion Engine
Engineering Fundamentals of the Internal Combustion Engine . I Willard W. Pulkrabek University of Wisconsin-· .. Platteville vi Contents 2-3 Mean Effective Pressure, 49 2-4 Torque and Power, 50 2-5 Dynamometers, 53 2-6 Air-Fuel Ratio and Fuel-Air Ratio, 55 2-7 Specific Fuel Consumption, 56 2-8 Engine Efficiencies, 59 2-9 Volumetric Efficiency, 60 , 2-10 Emissions, 62 2-11 Noise Abatement, 62 2-12 Conclusions-Working Equations, 63 Problems, 65 Design Problems, 67 3 ENGINE CYCLES 68 3-1 Air-Standard Cycles, 68 3-2 Otto Cycle, 72 3-3 Real Air-Fuel Engine Cycles, 81 3-4 SI Engine Cycle at Part Throttle, 83 3-5 Exhaust Process, 86 3-6 Diesel Cycle, 91 3-7 Dual Cycle, 94 3-8 Comparison of Otto, Diesel, and Dual Cycles, 97 3-9 Miller Cycle, 103 3-10 Comparison of Miller Cycle and Otto Cycle, 108 3-11 Two-Stroke Cycles, 109 3-12 Stirling Cycle, 111 3-13 Lenoir Cycle, 113 3-14 Summary, 115 Problems, 116 Design Problems, 120 4 THERMOCHEMISTRY AND FUELS 121 4-1 Thermochemistry, 121 4-2 Hydrocarbon Fuels-Gasoline, 131 4-3 Some Common Hydrocarbon Components, 134 4-4 Self-Ignition and Octane Number, 139 4-5 Diesel Fuel, 148 4-6 Alternate Fuels, 150 4-7 Conclusions, 162 Problems, 162 Design Problems, 165 Contents vii 5 AIR AND FUEL INDUCTION 166 5-1 Intake Manifold, 166 5-2 Volumetric Efficiency of SI Engines, 168 5-3 Intake Valves, 173 5-4 Fuel Injectors, 178 5-5 Carburetors, 181 5-6 Supercharging and Turbocharging, 190 5-7 Stratified Charge Engines and Dual Fuel Engines, 195 5-8 Intake for Two-Stroke Cycle Engines, 196 5-9 Intake for CI Engines, 199