Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLOWERS Herbaceous Perennials No
G A R D E N I N G S E R I E S FLOWERS Herbaceous Perennials no. 7.405 by M. Meehan, J.E. Klett and R.A. Cox 1 An ever-expanding palette of perennials lets home gardeners create showy collections of herbaceous perennials. Quick Facts... Under normal growing conditions, perennials live many years, dying back to the ground each winter. They quickly establish These herbaceous perennials themselves in a few growing seasons and create a are best adapted for Colorado’s backbone for the flower garden. lower elevations. Plants vary in flower color, bloom time, height, foliage texture and environmental Herbaceous perennials differ requirements. Environmental requirements include in bloom period, flower color, sun exposure, soil conditions and water needs. height, foliage texture and The key to a successful perennial garden is to environmental requirements. choose plants whose requirements match your site’s conditions. Environmental requirements Table 1 lists perennials adapted to the broad include sun and wind exposure range of growing conditions in Colorado’s lower or lack of it, soil conditions and elevations. Many also do well at higher elevations, but water needs. for a more specific listing of higher elevation perennials, see fact sheet 7.406, Flowers for Mountain Communities. Matching the perennial plant to More information on design and maintenance of perennial the site conditions produces a gardens can be found in 7.402, Perennial Gardening. Also see 7.840, Vegetable Garden: Soil Management and Fertilization. successful perennial garden. Key to Table 1: a only most common cultivars are listed. b Not Important. -

Bright Spots Plant Material Location
Bright Spots A SELF GUIDED WALKING TOUR September 13, 2017 Did you know…? Usually when we think of azaleas we think of Spring, but there are some beauties abloom in the garden right now. These are reblooming azaleas. They have already produced Spring flowers for Virginia’s Garden Week, and now they are contributing to Fall color. Many of these plants are patented under the name Encore® azalea. They first became available in the late 1990s, the work of Robert E. “Buddy” Lee, a plant breeder and nurseryman from Louisiana. This group derives from a cross between spring blooming azaleas and Rhododendron oldhamii “Fourth of July”. The garden’s Plant Explorer database lists 22 varieties of Encore® azaleas. Many have the word “Autumn” in their cultivar name. Several are in bloom now, including ‘Autumn Royalty’ and ‘Autumn Sundance’ listed below. Not much published material is available on these rebloomers, but the article by Will Ferrell (not the actor) in The Azalean (below) is a knowledgeable evaluation of the merits of many specific cultivars. Besides the Encore® series there are at least two other patented reblooming lines: ReBLOOM™ Azaleas by breeder Bob Head and Bloom-a-thon from Monrovia. To sow confusion, I must note that the Azalea ‘August to Frost’ with its bright white blossoms (along the Flagler walk) is not considered a rebloomer. It was hybridized by M. B. Matlack in 1940, possibly a cross between R. mucronatum var. mucronatum and an unknown species. Sources: http://www.encoreazalea.com/; “Personal Observations on Encore® Azaleas in a Zone 7 Garden”, Will Ferrell, The Azalean, Fall 2013, pp. -

5. KALIDIUM Moquin-Tandon in Candolle, Prodr. 13(2): 46, 146
Flora of China 5: 355-356. 2003. 5. KALIDIUM Moquin-Tandon in Candolle, Prodr. 13(2): 46, 146. 1849. 盐爪爪属 yan zhua zhua shu Shrubs small, much branched; branches not jointed. Leaves alternate, terete or undeveloped, fleshy, basally decurrent. Inflorescence pedunculate, spicate. Flowers spirally arranged, (1 or)3 borne in axil of a fleshy bract, appearing sunken into fleshy rachis, without bractlets, bisexual. Perianth 4- or 5-lobed, spongy in fruit, flat on top surface. Stamens 2. Ovary ovoid; stigmas 2, papillate. Fruit a utricle, enclosed by perianth. Seed vertical, compressed; testa subleathery; embryo semi-annular; perisperm present. Five species: C and SW Asia, SE Europe; five species in China. 1a. Leaves 4–10 mm; spikes 3–4 mm in diam. .................................................................................................................... 1. K. foliatum 1b. Leaves less than 3 mm or undeveloped; spikes 1.5–3 mm in diam. 2a. Branchlets slender; flowers 1 per bract ..................................................................................................................... 5. K. gracile 2b. Branchlets stout; flowers 3 per bract. 3a. Leaves developed, 1–3 mm, ovate, adaxially curved, apex acute ............................................................... 2. K. cuspidatum 3b. Leaves undeveloped, tuberculate, less than 1 mm, apex obtuse. 4a. Plants 10–25 cm tall, branched from base; leaves of branchlets narrow and obconic at base ......... 3. K. schrenkianum 4b. Plants 20–70 cm tall, branched from middle; leaves of branchlets sheathing at base ............................ 4. K. caspicum 1. Kalidium foliatum (Pallas) Moquin-Tandon in Candolle, 1b. Leaves 1–1.5 mm; plants densely Prodr. 13(2): 147. 1849. branched .................................................... 2b. var. sinicum 盐爪爪 yan zhua zhua 2a. Kalidium cuspidatum var. cuspidatum Salicornia foliata Pallas, Reise Russ. Reich. 1: 482. -

Effect of Achillea Millefolium Strips And
& Herpeto gy lo lo gy o : h C it u n r r r e Almeida, et al., Entomol Ornithol Herpetol 2017, 6:3 O n , t y R g e o l Entomology, Ornithology & s DOI: 10.4172/2161-0983.1000199 o e a m r o c t h n E ISSN: 2161-0983 Herpetology: Current Research Research Article Open Access Effect of Achillea millefolium Strips and Essential Oil on the European Apple Sawfly, Hoplocampa testudinea (Hymenoptera: Tenthredinidea) Jennifer De Almeida1, Daniel Cormier2* and Éric Lucas1 1Département des Sciences Biologiques, Université du Québec à Montréal, Montréal, Canada 2Research and Development Institute for the Agri-Environment, 335 rang des Vingt-Cinq Est, Saint-Bruno-de-Montarville, Qc, Canada *Corresponding author: Daniel Cormier, Research and Development Institute for the Agri-Environment, 335 rang des Vingt-Cinq Est, Saint-Bruno-de-Montarville, Qc, J3V 0G7, Canada, Tel: 450-653-7368; Fax: 653-1927; E-mail: [email protected] Received date: August 15, 2017; Accepted date: September 05, 2017; Published date: September 12, 2017 Copyright: © 2017 Almeidal JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract The European apple sawfly Hoplocampa testudinea (Klug) (Hymenoptera: Tenthredinidae) is a pest in numerous apple orchards in eastern North America. In Quebec, Canada, the European apple sawfly can damage up to 14% of apples and growers use phosphate insecticide during the petal fall stage to control the pest. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -

CHENOPODIACEAE 藜科 Li Ke Zhu Gelin (朱格麟 Chu Ge-Ling)1; Sergei L
Flora of China 5: 351-414. 2003. CHENOPODIACEAE 藜科 li ke Zhu Gelin (朱格麟 Chu Ge-ling)1; Sergei L. Mosyakin2, Steven E. Clemants3 Herbs annual, subshrubs, or shrubs, rarely perennial herbs or small trees. Stems and branches sometimes jointed (articulate); indumentum of vesicular hairs (furfuraceous or farinose), ramified (dendroid), stellate, rarely of glandular hairs, or plants glabrous. Leaves alternate or opposite, exstipulate, petiolate or sessile; leaf blade flattened, terete, semiterete, or in some species reduced to scales. Flowers monochlamydeous, bisexual or unisexual (plants monoecious or dioecious, rarely polygamous); bracteate or ebracteate. Bractlets (if present) 1 or 2, lanceolate, navicular, or scale-like. Perianth membranous, herbaceous, or succulent, (1–)3–5- parted; segments imbricate, rarely in 2 series, often enlarged and hardened in fruit, or with winged, acicular, or tuberculate appendages abaxially, seldom unmodified (in tribe Atripliceae female flowers without or with poorly developed perianth borne between 2 specialized bracts or at base of a bract). Stamens shorter than or equaling perianth segments and arranged opposite them; filaments subulate or linear, united at base and usually forming a hypogynous disk, sometimes with interstaminal lobes; anthers dorsifixed, incumbent in bud, 2-locular, extrorse, or dehiscent by lateral, longitudinal slits, obtuse or appendaged at apex. Ovary superior, ovoid or globose, of 2–5 carpels, unilocular; ovule 1, campylotropous; style terminal, usually short, with 2(–5) filiform or subulate stigmas, rarely capitate, papillose, or hairy on one side or throughout. Fruit a utricle, rarely a pyxidium (dehiscent capsule); pericarp membranous, leathery, or fleshy, adnate or appressed to seed. Seed horizontal, vertical, or oblique, compressed globose, lenticular, reniform, or obliquely ovoid; testa crustaceous, leathery, membranous, or succulent; embryo annular, semi-annular, or spiral, with narrow cotyledons; endosperm much reduced or absent; perisperm abundant or absent. -

The Role of Starch in the Day/Night Re-Programming of Stomata in Plants with Crassulacean Acid Metabolism Natalia Hurtado Castan
The role of starch in the day/night re-programming of stomata in plants with Crassulacean Acid Metabolism Natalia Hurtado Castano Doctor of Philosophy School of Natural and Environmental Sciences February 2020 Declaration This thesis is submitted to Newcastle University for the degree of Doctor of Philosophy. The research detailed within was performed between the years 2015-2019 and it was supervised by Professor Anne Borland and Professor Jeremy Barnes. I certify that none of the material offered in this thesis has been previously submitted by me for a degree or any other qualification at this or any other university. Natalia Hurtado Castano ii Abstract Crassulacean acid metabolism (CAM) is a specialised type of photosynthesis characterised by the unique inverted stomatal rhythm, which increases water use efficiency (WUE) and enhances the potential for sustainable biomass production in warmer and drier conditions. Starch turnover in the mesophyll of CAM species supports nocturnal CO2 assimilation and CAM activity. In C3 plants, starch metabolism has been reported to play an important role in determining stomatal behaviour; in this case, guard cell starch degradation is triggered by blue light, producing osmolytes that promotes stomatal opening. Based on the importance of starch and the little knowledge regarding CAM stomatal behaviour, this study tested the hypothesis that starch metabolism has been re-programmed in CAM plants to enable nocturnal stomatal opening, by using biochemical and genetic characterisation of wild type and RNAi lines with curtailed starch metabolism in the constitutive CAM species Kalanchoë fedtschenkoi. Measurements of guard cell starch content over 24 hours in wild type plants of K. -

Noteworthy Collections
48 THE MICHIGAN BOTANIST Vol. 52 NOTEWORTHY COLLECTIONS MICHIGAN Viburnum sieboldii Miq. Adoxaceae Siebold’s Arrowwood. Significance of the Report. First known naturalized occurrence in Michigan of a potentially invasive species. Previous Knowledge. Viburnum sieboldii is a large shrub or small tree native to Japan, where it occurs in thickets in lowlands and low mountains (Ohwi 1965). It was introduced to eastern North America in the late nineteenth century as a landscape ornamental, and is known to escape occasionally from cultivation in its introduced range (Kunstler 1993, Gleason and Cronquist 1991). Prior to this collection, naturalized populations of V. sieboldii were known in North America primarily from the Mid-Atlantic region of the United States, from Vir - ginia north to New York and Massachusetts (USDA NRCS 2014), although more recently it has also been reported from eastern and southern Ohio (Vincent et al. 2011). Escaped populations are known to establish in a variety of habitats, in - cluding mesic forests, stream edges, and suburban parks, and have been reported as abundant or invasive in New Jersey, New York, and Pennsylvania (Kunstler 1993, DeCandido and Lamont 2004, Pennsylvania Department of Conservation and Natural Resources 2014, Central New Jersey Invasive Species Strike Team 2014). On the other hand, Widrlechner and Iles (2002) found that V. sieboldii was at relatively low risk of naturalizing in Iowa because of climatic differences between there and the native habitat of V. sieboldii in Japan. However, it is un - known whether or how ornamental cultivars may differ from native genotypes in invasive potential. The genus Viburnum was long included in the Caprifoliaceae, but is now considered to belong to the Adoxaceae. -

ISTA List of Stabilised Plant Names 7Th Edition
ISTA List of Stabilised Plant Names 7th Edition ISTA Nomenclature Committee Chair Dr. M. Schori Published by All rights reserved. No part of this publication may be The International Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted in Richtiarkade 18, CH- 8304 Wallisellen, Switzerland any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2021 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 Valid from: 16.06.2021 ISTA List of Stabilised Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 7th Edition 2 ISTA List of Stabilised Plant Names Table of Contents A .............................................................................................................................................................. 7 B ............................................................................................................................................................ 21 C ........................................................................................................................................................... -
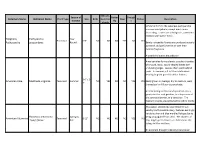
Common Name Botanical Name Alleghany
Attracts Season of Butter Drough Common Name Botanical Name Plant Type Size Birds Hummin Deer Native Description Interest fly t g birds Similar in form to the Japanese pachysandra one sees everywhere, except much more interesting. Leaves are a dull green, sometimes mottled with lighter flecks. Alleghany Pachysandra Year Perennial 6-8" NO NO NO YES NO YES Pachysandra procumbens Round Barely noticeable flowers are produced as early as March and perfume the air with their delicate fragrance. A wonderful native groundcover. American aloe forms a lovely succulent rosette of smooth, waxy, sword-shaped leaves with undulating edges. Leaves often sport reddish spots. In summer, a 3 to 5 foot stalk arises bearing fragrant greenish-white flowers. 3-6' x 2- American Aloe Manfreda virginica Perennial Summer NO YES NO NO YES YES Easily grown in average, dry to medium, well- 3' drained soil in full sun to part shade. An interesting architectural specimen, it is a good plant for rock gardens, in a dry corner of the perennial border, or a container. The fragrant blooms are pollinated by sphinx moths. This native, selected by Dale Hendrick's at nearby North Creek Nursery, features excitingly variable silver and blue marbled foliage due to Heuchera americana Spring to being propagated from seed. The clusters of American Alumroot Perennial 8-12" NO NO NO NO YES NO 'Dale's Strain' Fall tiny, bright green flowers are held above the foliage in May and June. An excellent drought tolerant groundcover. Viburnum trilobum is a native deciduous shrub to the northeastern and northwestern United States. -

PLANT in the SPOTLIGHT Cover of Ajuga in This Vignette at Pennsylvania's Chanticleer Garden
TheThe AmericanAmerican gardenergardener® TheThe MagazineMagazine ofof thethe AmericanAmerican HorticulturalHorticultural SocietySociety March / April 2013 Ornamental Grasses for small spaces Colorful, Flavorful Heirloom Tomatoes Powerhouse Plants with Multi-Seasonal Appeal Build an Easy Bamboo Fence contents Volume 92, Numbe1' 2 . March / Apl'il 2013 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS' FORUM 8 NEWS FROM THE AHS The AHS Encyclopediao/Gardening Techniques now available in paperback, the roth Great Gardens and Landscaping Symposium, registration opening soon for the National Children & Youth Garden Symposium, River Farm to participate in Garden Club of Virginia's Historic Garden Week II AHS NEWS SPECIAL Highlights from the AHS Travel Study Program trip to Spain. 12 AHS MEMBERS MAKING A DIFFERENCE Eva Monheim. 14 2013 GREAT AMERICAN GARDENERS AWARDS Meet this year's award recipients. 44 GARDEN SOLUTIONS Selecting disease-resistant plants. 18 FRAGRANT FLOWERING SHRUBS BY CAROLE OTTESEN Shrubs that bear fragrant flowers add an extra-sensory dimension 46 HOMEGROWN HARVEST to your landscape. Radish revelations. 48 TRAVELER'S GUIDE TO GARDENS 24 BUILD A BAMBOO FENCE BY RITA PELCZAR Windmill Island Gardens in Michigan. This easy-to-construct bamboo fence serves a variety of purposes and is attractive to boot. 50 BOOK REVIEWS No Nomeme VegetableGardening, The 28 GREAT GRASSES FOR SMALL SPACES BY KRIS WETHERBEE 2o-Minute Gardener, and World'sFair Gardem. Add texture and motion to your garden with these grasses and 52 GARDENER'S NOTEBOOK grasslike plants ideal for small sites and containers. Solomon's seal is Perennial Plant Association's 20I3 Plant of the Year, research shows plants 34 A SPECTRUM OF HEIRLOOM TOMATOES BY CRAIG LEHOULLIER may be able to communicate with each other, industry groups OFA and ANLAto If you enjoy growing heirloom tomatoes, you'll appreciate this consolidate, the Garden Club of America useful guide to some of the tastiest selections in a wide range of celebrates roo years, John Gaston Fairey colors. -

Ecological Ranges of Plant Species in the Monsoon Zone of the Russian Far East
In: Horizons in Earth Science Research, Volume 3 ISBN: 978-1-61122-197-8 Editors: Benjamin Veress and Jozsi Szigethy © 2011 Nova Science Publishers, Inc. The exclusive license for this PDF is limited to personal website use only. No part of this digital document may be reproduced, stored in a retrieval system or transmitted commercially in any form or by any means. The publisher has taken reasonable care in the preparation of this digital document, but makes no expressed or implied warranty of any kind and assumes no responsibility for any errors or omissions. No liability is assumed for incidental or consequential damages in connection with or arising out of information contained herein. This digital document is sold with the clear understanding that the publisher is not engaged in rendering legal, medical or any other professional services. Chapter 2 ECOLOGICAL RANGES OF PLANT SPECIES IN THE MONSOON ZONE OF THE RUSSIAN FAR EAST Vitaly P. Seledets1* and Nina S. Probatova2 1Pacific Institute of Geography FEB RAS, 690041 Vladivostok, Russia 2Institute of Biology and Soil Science FEB RAS, 690022 Vladivostok, Russia ABSTRACT The monsoon zone covers a considerable part of the Russian Far East (RFE), which includes the Kamchatka Peninsula, Sakhalin, the Kurile Islands, the continental coasts and islands of the Bering Sea, the Sea of Okhotsk, the Sea of Japan, and the Amur River basin. The problem of biodiversity in the monsoon zone is connected to species adaptations, speciation and florogenesis, the formation of plant communities, vegetation dynamics, and population structure. Our concept of the ecological range (ecorange, ER) of plant species (Seledets & Probatova 2007b) is aimed at adaptive strategies in the RFE monsoon zone compared with Inner Asia.