Antimigraine Agents, Other
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medication Guide
1 Medication Guide REYVOW® (RAY-vow) (lasmiditan) tablets, for oral use, CV What is the most important information I should know about REYVOW? • Do not drive or operate machinery for at least 8 hours after you take REYVOW, even if you feel well enough. • You should not take REYVOW if you cannot wait at least 8 hours between taking REYVOW and driving or operating machinery. What is REYVOW? REYVOW is a prescription medicine used for the acute treatment of migraine attacks with or without aura in adults. • REYVOW is not used as a preventive treatment of migraine. • It is not known if REYVOW is safe and effective in children. • REYVOW is a federally controlled substance (CV) because it contains lasmiditan that can be abused. Keep REYVOW in a safe place to protect it from theft. Never give your REYVOW to anyone else, because it may harm them. Selling or giving away REYVOW is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs. Before you take REYVOW, tell your healthcare provider about all of your medical conditions, including if you: • have liver problems • have high blood pressure • have a low heart rate • are allergic to lasmiditan • are pregnant or plan to become pregnant. It is not known if REYVOW will harm your unborn baby. • are breastfeeding or plan to breastfeed. It is not known if REYVOW passes into your breastmilk. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

New Drug Update: Not All That Glitters Is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018
9/23/2018 New Drug Update: Not All That Glitters is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018 PRESENTERS: Boise VA Medical Center Idaho State University PGY2 Ambulatory Care Residents: PGY2 Pharmacotherapy Resident: Audra Wilson, PharmD; Kat Liu, PharmD; Kailee Morton, PharmD Nitz Bankova, PharmD St. Luke’s Health System Infectious Disease Pharmacy Fellow: PGY2 Oncology Pharmacy Residents: Benjamin Pontefract, PharmD Andrew Li, PharmD; Amanda Wright, PharmD; Bryce Benson, PharmD PGY2 Mental Health Pharmacy Resident: Samantha Patton, PharmD Disclosures No conflicts of interest to disclose Learning Objectives • Describe clinical scenarios where newly approved medications are beneficial. • Compare newly approved medications with current standards of care. • Identify investigational new drugs and their potential clinical applications. 1 9/23/2018 Overview of Topics • Infectious Disease • Ambulatory Care • Diabetes Mellitus • Women’s Health • Anticoagulation • Pain Management • Oncology • Acute Myeloid Leukemia (AML) Infectious Disease: Plazomicin Delafloxacin Benjamin Pontefract, PharmD Plazomicin (Zemdri®):Overview • Systemic antibiotic in the aminoglycoside family • MoA: disrupts the 30s ribosome to prevent protein synthesis • Concentration dependent bactericidal activity • Approved to treat UTIs caused by E. coli, K. pneumoniae, Proteus spp, and Enterobacter cloaecae 2 9/23/2018 Aminoglycoside Shortcomings Toxicities Resistance • Nephrotoxicity • Decreased cell permeability • Ototoxicity • Altered ribosome binding sites • Therapeutic drug monitoring • AMG‐modifying enzymes (AME) Plazomicin: Efficacy • Study 009: • RCT comparing plazomicin IV to meropenem IV for cUTI caused by enterobactereceae • Plazomicin group was non‐inferior to meropenem group • Study 007: • RCT comparing plazomicin IV to polymyxin E IV for BSI cause by carbapenem‐resistant Enterobacteriaceae (CRE) • Plazomicin was associated with less mortality compared to polymyxin E Connolly L, Achaogen, Inc. -

Lasmiditan Is an Effective Acute Treatment for Migraine: a Phase 3 Randomized Study Bernice Kuca, Stephen D
ARTICLE OPEN ACCESS CLASS OF EVIDENCE Lasmiditan is an effective acute treatment for migraine A phase 3 randomized study Bernice Kuca, BA, MS, Stephen D. Silberstein, MD, Linda Wietecha, BSN, MS, Paul H. Berg, MS, Correspondence Gregory Dozier, MPH, and Richard B. Lipton, MD, on behalf of the COL MIG-301 Study Group Ms. Wietecha [email protected] Neurology® 2018;91:e2222-e2232. doi:10.1212/WNL.0000000000006641 Abstract RELATED ARTICLE Objective Article To assess the efficacy and safety of lasmiditan in the acute treatment of migraine. Galcanezumab in chronic migraine: The randomized, Methods double-blind, placebo- Adult patients with migraine were randomized (1:1:1) to a double-blind dose of oral lasmiditan controlled REGAIN study 200 mg, lasmiditan 100 mg, or placebo and were asked to treat their next migraine attack within Page 1082 4 hours of onset. Over 48 hours after dosing, patients used an electronic diary to record headache pain and the presence of nausea, phonophobia, and photophobia, one of which was MORE ONLINE designated their most bothersome symptom (MBS). Class of Evidence Criteria for rating Results ≥ therapeutic and diagnostic Of the 1,856 patients who treated an attack, 77.9% had 1 cardiovascular risk factors in addition studies to migraine. Compared with placebo, more patients dosed with lasmiditan 200 mg were free of NPub.org/coe headache pain at 2 hours after dosing (32.2% vs 15.3%; odds ratio [OR] 2.6, 95% confidence interval [CI] 2.0–3.6, p< 0.001), similar to those dosed with lasmiditan 100 mg (28.2%; OR 2.2, 95% CI 1.6–3.0, p< 0.001). -

NCT04179474 Study ID: 3110‐108‐002 Title: A
NCT04179474 Study ID: 3110‐108‐002 Title: A Phase 1b, Two‐Part, Open‐Label, Fixed‐Sequence, Safety, Tolerability and Drug‐Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Date: 14Aug2019 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Title Page Protocol Title: A Phase 1b, Two-Part, Open-Label, Fixed-Sequence, Safety, Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Number: 3110-108-002 Product: Ubrogepant (AGN-241688, MK-1602) Brief Protocol Title: Drug-Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab Study Phase: 1b Sponsor Name and Legal Registered Address: Allergan Pharmaceuticals International Limited, Clonshaugh Industrial Estate, Coolock, Dublin 17, Ireland US Agent: Allergan Sales, LLC, 2525 Dupont Dr, Irvine, California 92612, USA Regulatory Agency Identifying Number(s): IND #113924 SAE Reporting Fax Number/Email: Business Unit Email Phone Fax Allergan Approval Date: 14 August 2019 1 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Sponsor Signatories: Date Date The signatures of the sponsor signatories are collected on the protocol approval page. 2 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Table of Contents Title Page .........................................................................................................................................1 -

Acute Migraine Treatment
Acute Migraine Treatment Morris Levin, MD Professor of Neurology Director, Headache Center UCSF Department of Neurology San Francisco, CA Mo Levin Disclosures Consulting Royalties Allergan Oxford University Press Supernus Anadem Press Amgen Castle Connolly Med. Publishing Lilly Wiley Blackwell Mo Levin Disclosures Off label uses of medication DHE Antiemetics Zolmitriptan Learning Objectives At the end of the program attendees will be able to 1. List all important options in the acute treatment of migraine 2. Discuss the evidence and guidelines supporting the major migraine acute treatment options 3. Describe potential adverse effects and medication- medication interactions in acute migraine pharmacological treatment Case 27 y/o woman has suffered ever since she can remember from “sick headaches” . Pain is frontal, increases over time and is generally accompanied by nausea and vomiting. She feels depressed. The headache lasts the rest of the day but after sleeping through the night she awakens asymptomatic 1. Diagnosis 2. Severe Headache relief Diagnosis: What do we need to beware of? • Misdiagnosis of primary headache • Secondary causes of headache Red Flags in HA New (recent onset or change in pattern) Effort or Positional Later onset than usual (middle age or later) Meningismus, Febrile AIDS, Cancer or other known Systemic illness - Neurological or psych symptoms or signs Basic principles of Acute Therapy of Headaches • Diagnose properly, including comorbid conditions • Stratify therapy rather than treat in steps • Treat early -
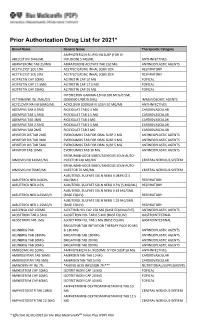
Prior Authorization Drug List for 2021*
Prior Authorization Drug List for 2021* Brand Name Generic Name Therapeutic Category AMPHOTERICIN B LIPID INJ SUSP (FOR IV ABELCET INJ 5MG/ML INFUSION) 5 MG/ML ANTI-INFECTIVES ABIRATERONE TAB 250MG ABIRATERONE ACETATE TAB 250 MG ANTINEOPLASTIC AGENTS ACETYLCYST SOL 10% ACETYLCYSTEINE INHAL SOLN 10% RESPIRATORY ACETYLCYST SOL 20% ACETYLCYSTEINE INHAL SOLN 20% RESPIRATORY ACITRETIN CAP 10MG ACITRETIN CAP 10 MG TOPICAL ACITRETIN CAP 17.5MG ACITRETIN CAP 17.5 MG TOPICAL ACITRETIN CAP 25MG ACITRETIN CAP 25 MG TOPICAL INTERFERON GAMMA-1B INJ 100 MCG/0.5ML ACTIMMUNE INJ 2MU/0.5 (2000000 UNIT/0.5ML) IMMUNOLOGIC AGENTS ACYCLOVIR NA INJ 50MG/ML ACYCLOVIR SODIUM IV SOLN 50 MG/ML ANTI-INFECTIVES ADEMPAS TAB 0.5MG RIOCIGUAT TAB 0.5 MG CARDIOVASCULAR ADEMPAS TAB 1.5MG RIOCIGUAT TAB 1.5 MG CARDIOVASCULAR ADEMPAS TAB 1MG RIOCIGUAT TAB 1 MG CARDIOVASCULAR ADEMPAS TAB 2.5MG RIOCIGUAT TAB 2.5 MG CARDIOVASCULAR ADEMPAS TAB 2MG RIOCIGUAT TAB 2 MG CARDIOVASCULAR AFINITOR DIS TAB 2MG EVEROLIMUS TAB FOR ORAL SUSP 2 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 3MG EVEROLIMUS TAB FOR ORAL SUSP 3 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 5MG EVEROLIMUS TAB FOR ORAL SUSP 5 MG ANTINEOPLASTIC AGENTS AFINITOR TAB 10MG EVEROLIMUS TAB 10 MG ANTINEOPLASTIC AGENTS ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 140MG/ML INJECTOR 140 MG/ML CENTRAL NERVOUS SYSTEM ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 70MG/ML INJECTOR 70 MG/ML CENTRAL NERVOUS SYSTEM ALBUTEROL SULFATE SOLN NEBU 0.083% (2.5 ALBUTEROL NEB 0.083% MG/3ML) RESPIRATORY ALBUTEROL NEB 0.5% ALBUTEROL SULFATE -

Acute Treatments for Migraine: Effectiveness and Value
Acute Treatments for Migraine: Effectiveness and Value Modeling Analysis Plan September 24, 2019 Institute for Clinical and Economic Review ©Institute for Clinical and Economic Review, 2019 Table of Contents 1. Approach ............................................................................................................................................ 3 2. Methods ............................................................................................................................................. 3 2.1 Overview and Model Structure .................................................................................................... 3 2.2 Key Model Choices and Assumptions .......................................................................................... 7 2.3 Populations .................................................................................................................................. 7 2.4 Interventions and Comparators ................................................................................................... 9 2.5 Input Parameters ......................................................................................................................... 9 2.6 Model Outcomes........................................................................................................................ 15 2.7 Model Analysis ........................................................................................................................... 16 References .......................................................................................................................................... -

Lasmiditan (Reyvow™) New Drug Update
Lasmiditan (Reyvow™) New Drug Update November 2019 Nonproprietary Name lasmiditan Brand Name Reyvow Manufacturer Eli Lilly and Company Form Tablets Strength 50 mg, 100 mg FDA Approval October 11, 2019 Market Availability Anticipated within 90 days of approval, following Drug Enforcement Agency (DEA) review FDA Approval Classification Standard Review FDB Classification- Specific TBD Therapeutic Class (HIC3) INDICATION1 Lasmiditan (Reyvow) is a serotonin 5-HT1F receptor agonist indicated for the acute treatment of migraine with or without aura in adults. Limitation of Use: Lasmiditan is not indicated for preventive treatment of migraine. PHARMACOKINETICS After oral administration of lasmiditan, the median time to maximum serum concentration (Tmax) is 1.8 hours. Food, age, gender, race, ethnicity, body weight, or renal function do not have a clinically significant effect on the pharmacokinetics of lasmiditan. The agent is 55% to 60% plasma protein bound. Lasmiditan is primarily metabolized via hepatic and extrahepatic metabolism, primarily by non- cytochrome P450 enzymes (e.g., ketone reduction) and has no pharmacologically active metabolites. Its mean terminal half-life is 5.7 hours. CONTRAINDICATIONS/WARNINGS Lasmiditan does not have any contraindications. Lasmiditan has been associated with significant impairment in the ability to drive or operate machinery following a single dose. Patients should be advised not take lasmiditan unless they are able to avoid driving or operating machinery for at least 8 hours after each lasmiditan dose. Prescribers should be aware that the patient’s ability to determine their own mental alertness may also be affected and should discuss this with the patient. Proprietary Information. Restricted Access – Do not disseminate or copy without approval. -

Headache Management: Pharmacological Approaches
REVIEW Headache management: Pract Neurol: first published as 10.1136/practneurol-2015-001167 on 3 July 2015. Downloaded from pharmacological approaches Alex J Sinclair,1,2 Aaron Sturrock,2 Brendan Davies,3 Manjit Matharu4 ▸ Additional material is ABSTRACT be very rewarding for the clinician. The published online only. To view Headache is one of the most common conditions purpose of this article, the first of two please visit the journal online (http://dx.doi.org/10.1136/ presenting to the neurology clinic, yet a linked articles, is to provide an up-to-date practneurol-2015-001167). significant proportion of these patients are overview of the pharmacological manage- unsatisfied by their clinic experience. Headache ment of common headache disorders 1Department of Neurobiology, School of Clinical and can be extremely disabling; effective treatment is (as well as a limited number of non- Experimental Medicine, College not only essential for patients but is rewarding pharmaceutical strategies). of Medical and Dental Sciences, for the physician. In this first of two parts review The University of Birmingham, of headache, we provide an overview of Birmingham, UK THE COMMON PRIMARY HEADACHE 2Neurology Department, headache management, emerging therapeutic DISORDERS University Hospitals Birmingham strategies and an accessible interpretation of In European populations, the annual sex- NHS Trust, Queen Elizabeth clinical guidelines to assist the busy neurologist. Hospital Birmingham, adjusted prevalence for tension-type Birmingham, UK headache is 35%, for migraine is 38%, 3 Department of Neurology, Royal BACKGROUND but for cluster headache is only Stoke University Hospital, ’ 0.15%.45These three together comprise Stoke-on-Trent, UK Headache is listed among the WHO s 4Headache Group, Institute of major causes of disability with a global the most prevalent primary headache dis- Neurology, London, UK prevalence of 47% (symptoms occurring orders. -

761119Orig1s000 SUMMARY REVIEW
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761119Orig1s000 SUMMARY REVIEW Summary Review Summary Review Date February 21, 2020 Heather Fitter, MD From Nick Kozauer, MD Billy Dunn, MD Subject Summary Review BLA # 761119 Applicant Lundbeck Seattle BioPharmaceuticals, Inc. Date of Submission December 26, 2018 PDUFA Goal Date February 21, 2020 Proprietary Name Vyepti Established or Proper Names Eptinezumab Solution for intravenous (IV) injection (100 Dosage Form mg/mL) Applicant Proposed Prevention of migraine in adults Indication/Population 100 mg IV infusion every 3 months; 300 mg IV Applicant Proposed Dosing Regimen(s) infusion every 3 months Recommendation on Regulatory Action Approval Recommended Indication/Population Preventive treatment of migraine in adults 100 mg IV infusion every 3 months; 300 mg IV Recommended Dosing Regimen(s) infusion every 3 months 1 Reference ID: 4564908 Summary Review 1. Benefit-Risk Assessment Benefit-Risk Assessment Framework Benefit-Risk Integrated Assessment Eptinezumab is a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand, and prevents CGRP binding to its receptor. The applicant provided information supporting safety and efficacy in patients with both chronic migraine (i.e., at least 15 headache days/month, with features of migraine headache on at least 8 days/month) and episodic migraine (i.e., up to 14 migraine headache days/month). There are several FDA-approved drugs for the preventive treatment of migraine. Three drugs in the anti-CGRP class were approved recently for this indication: erenumab (May 2018), fremanezumab (September 2018), and galcanezumab (September 2018). These three drugs are given by subcutaneous (SC) administration either monthly or quarterly, while eptinezumab is given intravenously (IV) quarterly. -

Aimovig, INN-Erenumab
Part VI: Summary of the risk management plan Summary of risk management plan for Aimovig (Erenumab) This is a summary of the risk management plan (RMP) for Aimovig. The RMP details important risks of Aimovig, how these risks can be minimized, and how more information will be obtained about Aimovig’s risks and uncertainties (missing information). Aimovig’s summary of product characteristics (SmPC) and its package leaflet give essential information to healthcare professionals and patients on how Aimovig should be used. This summary of the RMP for Aimovig should be read in the context of all this information including the assessment report of the evaluation and its plain-language summary, all which is part of the European Public Assessment Report (EPAR). Important new concerns or changes to the current ones will be included in updates of Aimovig’s RMP. I. The medicine and what it is used for Aimovig is authorized for the prophylaxis of migraine in adults who have at least 4 migraine days per month (see SmPC for the full indication). It contains erenumab (a human IgG2 monoclonal antibody) as the active substance and it is given by sc injections. Further information about the evaluation of Aimovig’s benefits can be found in Aimovig’s EPAR, including in its plain-language summary, available on the EMA website, under the medicine’s webpage: http://www.ema.europa.eu/ema/index.jsp?curl=/pages/medicines/human/medicines/004447/hum an_med_002275.jsp. II. Risks associated with the medicine and activities to minimize or further characterize the risks Important risks of Aimovig, together with measures to minimize such risks and the proposed studies for learning more about Aimovig's risks, are outlined below in Table 2.