761119Orig1s000 SUMMARY REVIEW
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

New Drug Update: Not All That Glitters Is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018
9/23/2018 New Drug Update: Not All That Glitters is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018 PRESENTERS: Boise VA Medical Center Idaho State University PGY2 Ambulatory Care Residents: PGY2 Pharmacotherapy Resident: Audra Wilson, PharmD; Kat Liu, PharmD; Kailee Morton, PharmD Nitz Bankova, PharmD St. Luke’s Health System Infectious Disease Pharmacy Fellow: PGY2 Oncology Pharmacy Residents: Benjamin Pontefract, PharmD Andrew Li, PharmD; Amanda Wright, PharmD; Bryce Benson, PharmD PGY2 Mental Health Pharmacy Resident: Samantha Patton, PharmD Disclosures No conflicts of interest to disclose Learning Objectives • Describe clinical scenarios where newly approved medications are beneficial. • Compare newly approved medications with current standards of care. • Identify investigational new drugs and their potential clinical applications. 1 9/23/2018 Overview of Topics • Infectious Disease • Ambulatory Care • Diabetes Mellitus • Women’s Health • Anticoagulation • Pain Management • Oncology • Acute Myeloid Leukemia (AML) Infectious Disease: Plazomicin Delafloxacin Benjamin Pontefract, PharmD Plazomicin (Zemdri®):Overview • Systemic antibiotic in the aminoglycoside family • MoA: disrupts the 30s ribosome to prevent protein synthesis • Concentration dependent bactericidal activity • Approved to treat UTIs caused by E. coli, K. pneumoniae, Proteus spp, and Enterobacter cloaecae 2 9/23/2018 Aminoglycoside Shortcomings Toxicities Resistance • Nephrotoxicity • Decreased cell permeability • Ototoxicity • Altered ribosome binding sites • Therapeutic drug monitoring • AMG‐modifying enzymes (AME) Plazomicin: Efficacy • Study 009: • RCT comparing plazomicin IV to meropenem IV for cUTI caused by enterobactereceae • Plazomicin group was non‐inferior to meropenem group • Study 007: • RCT comparing plazomicin IV to polymyxin E IV for BSI cause by carbapenem‐resistant Enterobacteriaceae (CRE) • Plazomicin was associated with less mortality compared to polymyxin E Connolly L, Achaogen, Inc. -

Pharmacy Prior Authorization Grid ALTCS, and Pharmacy
Please Note: Refer to the other PA grids for applicable covered services that require PA. PA Grids: Medical, Behavioral Health, Pharmacy Prior Authorization Grid ALTCS, and Pharmacy. (Effective Date of Service 1/1/2021) Injectables that require Prior Authorization All chemotherapeutic drugs must be used for FDA-approved indications and/or in accordance with NCCN guidelines *Indicates prior authorization required if billed charges are greater than $400 PA Required HMO 13 HCPCS Short Description (BUCA- Code SNP) 90378 Respiratory Syncytial Virus Immune Globulin Yes C9036 Patisiran Yes C9047 Caplacizumab-yhdp Yes C9061 Teprotumumab-trbw Yes C9063 Eptinezumab-jjmr Yes C9131 Factor VIII antihemophilic factor pegylated-auci Yes C9132 Prothrombin Complex Concentrate (Human), Kcentra Yes C9133 Factor IX (Antihemophilic Factor, Recombinant), Rixibus Yes C9399 Mipomersen (Kynamro) Yes J0129 Abatacept Yes J0135 Adalimumab Yes J0178 Aflibercept Yes J0179 Brolucizumab-dbll, 1 mg Yes J0180 Agalsidase Beta Yes J0205 Alglucerase Yes J0215 Alefacept Yes J0220 Alglucosidase Alfa (Myozyme) Yes J0221 Alglucosidase Alfa (Lumizyme) Yes J0222 Patisiran, 0.1 mg Yes J0223 Givosiran 0.5 mg Yes J0256 Alpha 1-Proteinase Inhibitor Yes J0257 Alpha 1-Proteinase Inhibitor (Glassia) Yes J0275 Alprostadil Urethral Suppository Yes J0490 Belimumab Yes J0517 Benralizumab Yes J0567 Cerliponase alfa Yes J0570 Buprenorphine implant Yes J0584 Burosumab-twza 1 mg Yes J0585 Onabotulinumtoxina (Botox) Yes J0586 Abobotulinumtoxina (Dysport) Yes J0587 Rimabotulinumtoxinb (Myobloc) -

NCT04179474 Study ID: 3110‐108‐002 Title: A
NCT04179474 Study ID: 3110‐108‐002 Title: A Phase 1b, Two‐Part, Open‐Label, Fixed‐Sequence, Safety, Tolerability and Drug‐Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Date: 14Aug2019 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Title Page Protocol Title: A Phase 1b, Two-Part, Open-Label, Fixed-Sequence, Safety, Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Number: 3110-108-002 Product: Ubrogepant (AGN-241688, MK-1602) Brief Protocol Title: Drug-Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab Study Phase: 1b Sponsor Name and Legal Registered Address: Allergan Pharmaceuticals International Limited, Clonshaugh Industrial Estate, Coolock, Dublin 17, Ireland US Agent: Allergan Sales, LLC, 2525 Dupont Dr, Irvine, California 92612, USA Regulatory Agency Identifying Number(s): IND #113924 SAE Reporting Fax Number/Email: Business Unit Email Phone Fax Allergan Approval Date: 14 August 2019 1 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Sponsor Signatories: Date Date The signatures of the sponsor signatories are collected on the protocol approval page. 2 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Table of Contents Title Page .........................................................................................................................................1 -

Oxford Policy Update Bulletin: April 2020
Oxford April 2020 policy update bulletin Medical & Administrative Policy Updates Take Note POLICY IMPLEMENTATION DELAYED Implementation of the changes associated with the following Clinical Policies, previously announced for an effective date on or after Apr. 1, 2020, has been delayed as noted below: Policy Title Status Effective Date Genitourinary Pathogen Nucleic Acid Detection Panel Testing New May 1, 2020 Jun. 1, 2020 Outpatient Surgical Procedures - Site of Service Revised Apr. 6, 2020 TBD Tysabri® (Natalizumab) New Apr. 1, 2020 Jul. 1, 2020 Access a policy listed below for complete details on the latest updates. A comprehensive summary of changes is provided at the bottom of every policy document for your reference. To view a detailed version of this bulletin, click here. Policy Title Status Effective Date CLINICAL POLICY Abnormal Uterine Bleeding and Uterine Fibroids Revised Jun. 1, 2020 Actemra® (Tocilizumab) Injection for Intravenous Infusion Revised May 1, 2020 Adakveo® (Crizanlizumab-Tmca) Updated Apr. 1, 2020 Adakveo® (Crizanlizumab-Tmca) Revised Jul. 1, 2020 Cell-Free Fetal DNA Testing Revised Jun. 1, 2020 Cimzia® (Certolizumab Pegol) New Jul. 1, 2020 Collagen Crosslinks and Biochemical Markers of Bone Turnover Revised May 1, 2020 Drug Coverage Criteria – New and Therapeutic Equivalent Medications Revised May 1, 2020 Drug Coverage Guidelines Revised Apr. 1, 2020 Adakveo (Crizanlizumab-Tmca) Givlaari (Givosiran) Ziextenzo (Pegfilgrastim-Bmez) Drug Coverage Guidelines Revised May 1, 2020 Adlyxin (Lixisenatide) Arnuity -

Patient Focused Disease State and Assistance Programs
Patient Focused Disease State and Assistance Programs Medication Medication Toll-free Brand (Generic) Website number Additional Resources Allergy/Asthma Xolair (omalizumab) xolair.com 1-866-4-XOLAIR lung.org Cardiovascular Pradaxa (dabigatran) pradaxa.com 877-481-5332 heart.org Praluent (alirocumab) praluent.com 844-PRALUENT thefhfoundation.org Repatha (evolocumab) repatha.com 844-REPATHA Tikosyn (dofetilide) tikosyn.com 800-879-3477 Crohn’s Disease Cimzia (certolizumab pegol) cimzia.com 866-4-CIMZIA crohnsandcolitis.com Humira (adalimumab) humira.com 800-4-HUMIRA crohnsforum.com Stelara (ustekinumab) stelarainfo.com 877-STELARA Dermatology Cosentyx (secukinumab) cosentyx.com 844-COSENTYX psoriasis.org Dupixent (dupilumab) dupixent.com 844-DUPIXENT nationaleczema.org Enbrel (etanercept) enbrel.com 888-4-ENBREL Humira (adalimumab) humira.com 800-4-HUMIRA Otezla (apremilast) otezla.com 844-4-OTEZLA Stelara (ustekinumab) stelarainfo.com 877-STELARA Taltz (ixekizumab) taltz.com 800-545-5979 Hematology Aranesp (darbepoetin alfa) aranesp.com 805-447-1000 chemocare.com Granix (filgrastim) granixrx.com 888-4-TEVARX hematology.org Jadenu (deferasirox) jadenu.com 888-282-7630 Neulasta (pegfilgrastim) neulasta.com 800-77-AMGEN Neupogen (filgrastim) neupogen.com 800-77-AMGEN Nivestym (filgrastim) nivestym.com 800-879-3477 Zarxio (filgrastim) zarxio.com 800-525-8747 Zytiga (abiraterone) zytiga.com 800-JANSSEN Hepatitis B Baraclude (entecavir) baraclude.com 800-321-1335 cdc.gov Viread (tenofovir disoproxil viread.com 800-GILEAD-5 hepb.org fumarate) -

The Breakthrough of Biosimilars: a Twist in the Narrative of Biological Therapy
biomolecules Review The Breakthrough of Biosimilars: A Twist in the Narrative of Biological Therapy Eva Rahman Kabir 1,* , Shannon Sherwin Moreino 1 and Mohammad Kawsar Sharif Siam 1,2 1 Department of Pharmacy, BRAC University, Dhaka 1212, Bangladesh 2 Department of Chemistry, University of Cambridge, Cambridge CB2 1EW, UK * Correspondence: [email protected] Received: 12 June 2019; Accepted: 20 August 2019; Published: 24 August 2019 Abstract: The coming wave of patent expiries of first generation commercialized biotherapeutical drugs has seen the global market open its doors to close copies of these products. These near perfect substitutes, which are termed as “biosimilars”, do not need to undergo intense clinical trials for their approval. However, they are mandated to produce identical similarity from their reference biologics in terms of clinical safety and efficacy. As such, these biosimilar products promise to foster unprecedented access to a wide range of life-saving biologics. However, seeing this promise be fulfilled requires the development of biosimilars to be augmented with product trust, predictable regulatory frameworks, and sustainable policies. It is vital for healthcare and marketing professionals to understand the critical challenges surrounding biosimilar use and implement informed clinical and commercial decisions. A proper framework of pharmacovigilance, education, and scientific exchange for biologics and biosimilars would ensure a dramatic rise in healthcare access and market sustainability. This paper seeks to collate and review all relevant published intelligence of the health and business potential of biosimilars. In doing so, it provides a visualization of the essential steps that are required to be taken for global biosimilar acceptance. -
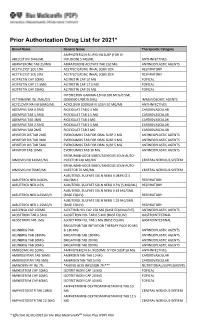
Prior Authorization Drug List for 2021*
Prior Authorization Drug List for 2021* Brand Name Generic Name Therapeutic Category AMPHOTERICIN B LIPID INJ SUSP (FOR IV ABELCET INJ 5MG/ML INFUSION) 5 MG/ML ANTI-INFECTIVES ABIRATERONE TAB 250MG ABIRATERONE ACETATE TAB 250 MG ANTINEOPLASTIC AGENTS ACETYLCYST SOL 10% ACETYLCYSTEINE INHAL SOLN 10% RESPIRATORY ACETYLCYST SOL 20% ACETYLCYSTEINE INHAL SOLN 20% RESPIRATORY ACITRETIN CAP 10MG ACITRETIN CAP 10 MG TOPICAL ACITRETIN CAP 17.5MG ACITRETIN CAP 17.5 MG TOPICAL ACITRETIN CAP 25MG ACITRETIN CAP 25 MG TOPICAL INTERFERON GAMMA-1B INJ 100 MCG/0.5ML ACTIMMUNE INJ 2MU/0.5 (2000000 UNIT/0.5ML) IMMUNOLOGIC AGENTS ACYCLOVIR NA INJ 50MG/ML ACYCLOVIR SODIUM IV SOLN 50 MG/ML ANTI-INFECTIVES ADEMPAS TAB 0.5MG RIOCIGUAT TAB 0.5 MG CARDIOVASCULAR ADEMPAS TAB 1.5MG RIOCIGUAT TAB 1.5 MG CARDIOVASCULAR ADEMPAS TAB 1MG RIOCIGUAT TAB 1 MG CARDIOVASCULAR ADEMPAS TAB 2.5MG RIOCIGUAT TAB 2.5 MG CARDIOVASCULAR ADEMPAS TAB 2MG RIOCIGUAT TAB 2 MG CARDIOVASCULAR AFINITOR DIS TAB 2MG EVEROLIMUS TAB FOR ORAL SUSP 2 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 3MG EVEROLIMUS TAB FOR ORAL SUSP 3 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 5MG EVEROLIMUS TAB FOR ORAL SUSP 5 MG ANTINEOPLASTIC AGENTS AFINITOR TAB 10MG EVEROLIMUS TAB 10 MG ANTINEOPLASTIC AGENTS ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 140MG/ML INJECTOR 140 MG/ML CENTRAL NERVOUS SYSTEM ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 70MG/ML INJECTOR 70 MG/ML CENTRAL NERVOUS SYSTEM ALBUTEROL SULFATE SOLN NEBU 0.083% (2.5 ALBUTEROL NEB 0.083% MG/3ML) RESPIRATORY ALBUTEROL NEB 0.5% ALBUTEROL SULFATE -

Alder Biopharmaceuticals, Inc. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-Q ☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended March 31, 2019 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 001-36431 Alder BioPharmaceuticals, Inc. (Exact name of registrant as specified in its charter) Delaware 90-0134860 (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) 11804 North Creek Parkway South Bothell, WA 98011 (Address of principal executive offices including zip code) Registrant’s telephone number, including area code: (425) 205-2900 Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐ Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. -

VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD
Proprietary Information of United Healthcare: The information contained in this document is proprietary and the sole property of United HealthCare Services, Inc. Unauthorized copying, use and distribution of this information are strictly prohibited. Copyright 2020 United HealthCare Services, Inc. UnitedHealthcare® Community Plan Medical Benefit Drug Policy VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD Table of Contents Page Commercial Policy APPLICATION ...................................................... 1 Vyepti™ (Eptinezumab) COVERAGE RATIONALE ........................................ 1 APPLICABLE CODES ............................................. 2 BACKGROUND ................................................... 43 CLINICAL EVIDENCE ............................................ 4 U.S. FOOD AND DRUG ADMINISTRATION ............ 54 CENTERS FOR MEDICARE AND MEDICAID SERVICES 54 REFERENCES ....................................................... 5 POLICY HISTORY/REVISION INFORMATION ...... 65 INSTRUCTIONS FOR USE .................................... 65 APPLICATION This Medical Benefit Drug Policy only applies to state of Louisiana. COVERAGE RATIONALE Vyepti has been added to the Review at Launch program. Please reference the Medical Benefit Drug Policy titled Review at Launch for New to Market Medications for additional details. Chronic Migraine Vyepti is proven and medically necessary for the preventive treatment of chronic migraines when all of the following criteria are met: For initial therapy, all of the -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

Aimovig, INN-Erenumab
Part VI: Summary of the risk management plan Summary of risk management plan for Aimovig (Erenumab) This is a summary of the risk management plan (RMP) for Aimovig. The RMP details important risks of Aimovig, how these risks can be minimized, and how more information will be obtained about Aimovig’s risks and uncertainties (missing information). Aimovig’s summary of product characteristics (SmPC) and its package leaflet give essential information to healthcare professionals and patients on how Aimovig should be used. This summary of the RMP for Aimovig should be read in the context of all this information including the assessment report of the evaluation and its plain-language summary, all which is part of the European Public Assessment Report (EPAR). Important new concerns or changes to the current ones will be included in updates of Aimovig’s RMP. I. The medicine and what it is used for Aimovig is authorized for the prophylaxis of migraine in adults who have at least 4 migraine days per month (see SmPC for the full indication). It contains erenumab (a human IgG2 monoclonal antibody) as the active substance and it is given by sc injections. Further information about the evaluation of Aimovig’s benefits can be found in Aimovig’s EPAR, including in its plain-language summary, available on the EMA website, under the medicine’s webpage: http://www.ema.europa.eu/ema/index.jsp?curl=/pages/medicines/human/medicines/004447/hum an_med_002275.jsp. II. Risks associated with the medicine and activities to minimize or further characterize the risks Important risks of Aimovig, together with measures to minimize such risks and the proposed studies for learning more about Aimovig's risks, are outlined below in Table 2.