Pharmacy Prior Authorization Grid ALTCS, and Pharmacy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

Novartis R&D and Investor Update
Novartis AG Investor Relations Novartis R&D and investor update November 5, 2018 Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” “agreement to acquire,” or similar expressions, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this presentation, or regarding potential future revenues from such products, or regarding the proposed acquisition of Endocyte, Inc. (Endocyte) by Novartis including the potential outcome and expected timing for completion of the proposed acquisition, and the potential impact on Novartis of the proposed acquisition, including express or implied discussions regarding potential future sales or earnings of Novartis, and any potential strategic benefits, synergies or opportunities expected as a result of the proposed acquisition. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this presentation will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. -

Oxford Policy Update Bulletin: April 2020
Oxford April 2020 policy update bulletin Medical & Administrative Policy Updates Take Note POLICY IMPLEMENTATION DELAYED Implementation of the changes associated with the following Clinical Policies, previously announced for an effective date on or after Apr. 1, 2020, has been delayed as noted below: Policy Title Status Effective Date Genitourinary Pathogen Nucleic Acid Detection Panel Testing New May 1, 2020 Jun. 1, 2020 Outpatient Surgical Procedures - Site of Service Revised Apr. 6, 2020 TBD Tysabri® (Natalizumab) New Apr. 1, 2020 Jul. 1, 2020 Access a policy listed below for complete details on the latest updates. A comprehensive summary of changes is provided at the bottom of every policy document for your reference. To view a detailed version of this bulletin, click here. Policy Title Status Effective Date CLINICAL POLICY Abnormal Uterine Bleeding and Uterine Fibroids Revised Jun. 1, 2020 Actemra® (Tocilizumab) Injection for Intravenous Infusion Revised May 1, 2020 Adakveo® (Crizanlizumab-Tmca) Updated Apr. 1, 2020 Adakveo® (Crizanlizumab-Tmca) Revised Jul. 1, 2020 Cell-Free Fetal DNA Testing Revised Jun. 1, 2020 Cimzia® (Certolizumab Pegol) New Jul. 1, 2020 Collagen Crosslinks and Biochemical Markers of Bone Turnover Revised May 1, 2020 Drug Coverage Criteria – New and Therapeutic Equivalent Medications Revised May 1, 2020 Drug Coverage Guidelines Revised Apr. 1, 2020 Adakveo (Crizanlizumab-Tmca) Givlaari (Givosiran) Ziextenzo (Pegfilgrastim-Bmez) Drug Coverage Guidelines Revised May 1, 2020 Adlyxin (Lixisenatide) Arnuity -

Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value
Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value Evidence Report Posted March 12, 2020 Updated February 9, 2021 Prepared for This evidence report was updated on February 9, 2021 with minor wording changes. ©Institute for Clinical and Economic Review, 2020 ICER Staff University of Calgary Pamela Bradt, MD, MPH Eldon Spackman, PhD Chief Scientific Officer Assistant Professor ICER Community Health Sciences & O'Brien Institute of Public Health University of Calgary Patricia G. Synnott, MALD, MS Director, Evidence Review ICER Rick Chapman, PhD, MS Director of Health Economics ICER Molly Beinfeld, MPH Research Lead, Evidence Synthesis ICER David M. Rind, MD, MSc Chief Medical Officer ICER Steven D. Pearson, MD, MSc President ICER The role of the University of Calgary is limited to the development of the cost-effectiveness model, and the resulting ICER reports do not necessarily represent the views of the University of Calgary. None of the above authors disclosed any conflicts of interest. How to cite this document: Bradt P, Spackman E, Synnott PG, Chapman R, Beinfeld M, Rind DM, Pearson SD. Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value. Institute for Clinical and Economic Review, January 23, 2020. https://icer.org/wp-content/uploads/2020/10/ICER_SCD_Evidence- Report_031220-FOR-PUBLICATION.pdf DATE OF PUBLICATION: March 12, 2020 Pamela Bradt served as the lead author for the report. Patricia Synnott led the systematic review and authorship of the comparative clinical effectiveness section in collaboration with Serina Herron-Smith and Avery McKenna. Patricia Synnott and Molly Beinfeld authored the chapter describing what ICER learned from patients (Chapter 2. -

Kalbitor® (Ecallantide)
Kalbitor® (ecallantide) (Subcutaneous) Document Number: MODA-0167 Last Review Date: 10/01/2020 Date of Origin: 08/27/2013 Dates Reviewed: 04/2014, 09/2014, 03/2015, 06/2015, 09/2015, 12/2015, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 06/2017, 09/2017, 12/2017, 03/2018, 06/2018 10/2018, 10/2019, 03/2020, 10/2020 I. Length of Authorization Coverage will be provided for 12 weeks and is eligible for renewal (unless otherwise specified). The cumulative amount of medication(s) the patient has on-hand, indicated for the acute treatment of HAE, will be taken into account when authorizing. The authorization will provide a sufficient quantity in order for the patient to have a cumulative amount of HAE medication on-hand in order to treat up to 4 acute attacks per 4 weeks for the duration of the authorization (unless otherwise specified). II. Dosing Limits A. Quantity Limit (max daily dose) [NDC unit]: Kalbitor 10 mg vial: 24 vials per 28 days B. Max Units (per dose and over time) [HCPCS Unit]: 240 billable units per 28 days III. Initial Approval Criteria 1 Coverage is provided in the following conditions: Patient is at least 12 years of age; AND Universal Criteria 1,13,18 Must be prescribed by, or in consultation with, a specialist in: allergy, immunology, hematology, pulmonology, or medical genetics; AND Confirmation the patient is avoiding the following possible triggers for HAE attacks: o Estrogen-containing oral contraceptive agents AND hormone replacement therapy; AND o Antihypertensive agents containing ACE inhibitors; AND o Dipeptidyl peptidase IV (DPP-IV) inhibitors (e.g., sitagliptin); AND o Neprilysin inhibitors (e.g., sacubitril) Moda Health Plan, Inc. -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -

The Use of Radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor Deficiency
Avances en Biomedicina ISSN: 2477-9369 ISSN: 2244-7881 [email protected] Universidad de los Andes Venezuela The use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Lara de la Rosa, María del Pilar; Conde Alcañiz, Amparo; Moreno Ramírez, David; Illescas Vacas, Ana; Guardia Martínez, Pedro The use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Avances en Biomedicina, vol. 7, no. 2, 2018 Universidad de los Andes, Venezuela Available in: https://www.redalyc.org/articulo.oa?id=331359393006 PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Casos Clínicos e use of radiotherapy in Hereditary Angioedema Type 1- C1 Inhibitor deficiency Uso de radioterapia en Angioedema hereditario por déficit de C1 inhibidor tipo I María del Pilar Lara de la Rosa [email protected] University Hospital Virgen Macarena, España Amparo Conde Alcañiz University Hospital Virgen Macarena, España David Moreno Ramírez University Hospital Virgen Macarena, España Ana Illescas Vacas University Hospital Virgen Macarena, España Pedro Guardia Martínez University Hospital Virgen Macarena, España Avances en Biomedicina, vol. 7, no. 2, 2018 Universidad de los Andes, Venezuela Received: 27 February 2018 Accepted: 21 June 2018 Abstract: We present a clinical case of a 72 year old man with Hereditary Angioedema Type 1. It´s a rare, potentially fatal disease, especially due to causing episodes of Redalyc: https://www.redalyc.org/ laryngeal angioedema. He has a past medical history of lip squamous-cell skin cancer, articulo.oa?id=331359393006 which is currently relapsing, with lateral margins of the surgical resection affected requiring treatment with local radiotherapy. -

Alder Biopharmaceuticals, Inc. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-Q ☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended March 31, 2019 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 001-36431 Alder BioPharmaceuticals, Inc. (Exact name of registrant as specified in its charter) Delaware 90-0134860 (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) 11804 North Creek Parkway South Bothell, WA 98011 (Address of principal executive offices including zip code) Registrant’s telephone number, including area code: (425) 205-2900 Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐ Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. -

VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD
Proprietary Information of United Healthcare: The information contained in this document is proprietary and the sole property of United HealthCare Services, Inc. Unauthorized copying, use and distribution of this information are strictly prohibited. Copyright 2020 United HealthCare Services, Inc. UnitedHealthcare® Community Plan Medical Benefit Drug Policy VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD Table of Contents Page Commercial Policy APPLICATION ...................................................... 1 Vyepti™ (Eptinezumab) COVERAGE RATIONALE ........................................ 1 APPLICABLE CODES ............................................. 2 BACKGROUND ................................................... 43 CLINICAL EVIDENCE ............................................ 4 U.S. FOOD AND DRUG ADMINISTRATION ............ 54 CENTERS FOR MEDICARE AND MEDICAID SERVICES 54 REFERENCES ....................................................... 5 POLICY HISTORY/REVISION INFORMATION ...... 65 INSTRUCTIONS FOR USE .................................... 65 APPLICATION This Medical Benefit Drug Policy only applies to state of Louisiana. COVERAGE RATIONALE Vyepti has been added to the Review at Launch program. Please reference the Medical Benefit Drug Policy titled Review at Launch for New to Market Medications for additional details. Chronic Migraine Vyepti is proven and medically necessary for the preventive treatment of chronic migraines when all of the following criteria are met: For initial therapy, all of the -
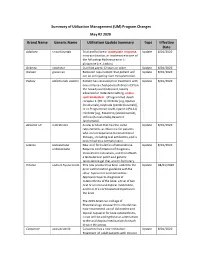
Summary of Utilization Management (UM) Program Changes May #2 2020
Summary of Utilization Management (UM) Program Changes May #2 2020 Brand Name Generic Name Utilization Update Summary Type Effective Date Adakveo crizanlizumab Trial and failure or inadequate response, Update 8/01/2020 contraindication, or intolerance to one of the following: Hydroxyurea or L- glutamine (i.e., Endari) Oxbryta voxelotor Clarified age to 12 years or older Update 8/01/2020 Givlaari givosiran Removed requirement that patient will Update 8/01/2020 not be anticipating liver transplantation. Padcev enfortumab vedotin Patient has received prior treatment with Update 8/01/2020 one immune checkpoint inhibitors (CPI) in the neoadjuvant/adjuvant, locally advanced or metastatic setting, unless contraindicated: i) Programmed death receptor-1 (PD-1) inhibitor [e.g.,Opdivo (nivolumab), Keytruda (pembrolizumab)], or ii) Programmed death-ligand 1 (PD-L1) inhibitor [e.g., Tecentriq (atezolizumab), Imfinzi (durvalumab), Bavencio (avelumab)]. Absorica LD isotretinoin A new product that has the same Update 8/01/2020 requirements as Absorica: for patients who are unresponsive to conventional therapy, including oral antibiotics, and is prescribed by a dermatologist. Jatenzo testosterone New oral formulation of testosterone. Update 8/01/2020 undecanoate Requires confirmation of diagnosis, testosterone lab values, and trial of both a testosterone patch and generic testosterone gel that are on formulary. Triluron sodium hyaluronate This new product has been added to the Update 08/01/2020 prior authorization guideline with the other hyaluronic acid derivatives. Approval requires diagnosis of osteoarthritis of the knee, a trial of two oral or an oral and topical medication, and trial of a corticosteroid injection in the knee. The 2019 American College of Rheumatology Osteoarthritis Guidelines now recommend use of duloxetine and topical capsaicin for knee osteoarthritis, so we will be adding these as alternatives to the oral/topical medications for each drug in this group. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities