P&T Summary 2Q2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

Denosumab Versus Romosozumab for Postmenopausal Osteoporosis Treatment
www.nature.com/scientificreports OPEN Denosumab versus romosozumab for postmenopausal osteoporosis treatment Tomonori Kobayakawa1, Akiko Miyazaki2, Makoto Saito3, Takako Suzuki2,4, Jun Takahashi2 & Yukio Nakamura2* Denosumab and romosozumab, a recently approved new drug, are efective and widely known molecular-targeted drugs for postmenopausal osteoporosis treatment. However, no studies have directly compared their therapeutic efects or safety in postmenopausal osteoporosis. This retrospective observational registry study compared the efcacy of 12-month denosumab or romosozumab treatment in postmenopausal osteoporosis patients. The primary outcome was the change in bone mineral density (BMD) at the lumbar spine. Secondary outcomes included BMD changes at the total hip and femoral neck, changes in bone turnover markers, and adverse events. Propensity score matching was employed to assemble patient groups with similar baseline characteristics. Sixty-nine patients each received either denosumab or romosozumab for 12 months. The mean 12-month percentage change from baseline in lumbar spine BMD was 7.2% in the denosumab group and 12.5% in the romosozumab group, indicating a signifcant diference between the groups. The percentage changes in BMD at both the total hip and femoral neck were also signifcantly higher at 12 months in the romosozumab group than in the denosumab group. In denosumab patients, bone formation and bone resorption markers were signifcantly decreased at 6 and 12 months from baseline. In the romosozumab group, the bone formation marker was signifcantly increased at 6 months and then returned to baseline, while the bone resorption marker was signifcantly decreased at both time points. Adverse events were few and predominantly minor in both groups, with no remarkable diference in the incidence of new vertebral fractures. -

Increased Bone Mineral Density for 1 Year of Romosozumab, Vs Placebo, Followed by 2 Years of Denosumab in the Japanese Subgroup
Archives of Osteoporosis (2019) 14:59 https://doi.org/10.1007/s11657-019-0608-z ORIGINAL ARTICLE Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension Akimitsu Miyauchi1 & Rajani V. Dinavahi2 & Daria B. Crittenden2 & Wenjing Yang2 & Judy C. Maddox2 & Etsuro Hamaya3 & Yoichi Nakamura3 & Cesar Libanati4 & Andreas Grauer2 & Junichiro Shimauchi3 Received: 1 March 2019 /Accepted: 18 May 2019 # The Author(s) 2019 Abstract Summary Romosozumab, which binds sclerostin, rebuilds the skeletal foundation before transitioning to antiresorptive treat- ment. This subgroup analysis of an international, randomized, double-blind study in postmenopausal women with osteoporosis showed efficacy and safety outcomes for romosozumab followed by denosumab in Japanese women were generally consistent with those for the overall population. Purpose In the international, randomized, double-blind, phase 3 FRActure study, in postmenopausal woMen with ostEoporosis (FRAME; NCT01575834), romosozumab followed by denosumab significantly improved bone mineral density (BMD) and reduced fracture risk. This report evaluates Japanese women in FRAME. Methods Postmenopausal women with osteoporosis (T-score − 3.5 to − 2.5 at total hip or femoral neck) received romosozumab 210 mg or placebo subcutaneously monthly for 12 months, then each group received denosumab 60 mg subcutaneously every 6 months for 24 months. The key endpoint for Japanese women was BMD change. Other endpoints included new vertebral, clinical, and nonvertebral fracture; the subgroup analysis did not have adequate power to demonstrate statistically significant reductions. Results Of 7180 enrolled subjects, 492 (6.9%) were Japanese (247 romosozumab, 245 placebo). -

Galcanezumab-Gnlm) Injection, for Subcutaneous Use
1 HIGHLIGHTS OF PRESCRIBING INFORMATION • Administer in the abdomen, thigh, back of the upper arm, or These highlights do not include all the information needed to use buttocks subcutaneously. (2.3) EMGALITY safely and effectively. See full prescribing information for EMGALITY. ---------------------- DOSAGE FORMS AND STRENGTHS -------------------- • Injection: 120 mg/mL solution in a single-dose prefilled pen (3) EMGALITY (galcanezumab-gnlm) injection, for subcutaneous use Initial U.S. Approval: 2018 • Injection: 120 mg/mL solution in a single-dose prefilled syringe (3) • Injection: 100 mg/mL solution in a single-dose prefilled syringe (3) --------------------------- RECENT MAJOR CHANGES ------------------------- Indications and Usage, Episodic Cluster Headache (1.2) 06/2019 ------------------------------- CONTRAINDICATIONS ----------------------------- Dosage and Administration, Recommended Dosing for Episodic EMGALITY is contraindicated in patients with serious hypersensitivity Cluster Headache (2.2) 06/2019 to galcanezumab-gnlm or to any of the excipients. (4) ---------------------------- INDICATIONS AND USAGE -------------------------- ------------------------ WARNINGS AND PRECAUTIONS ---------------------- EMGALITY® is a calcitonin-gene related peptide antagonist indicated in Hypersensitivity Reactions: If a serious hypersensitivity reaction adults for the: occurs, discontinue administration of EMGALITY and initiate appropriate therapy. Hypersensitivity reactions could occur days after • preventive treatment of migraine. (1.1) -

New Drug Update: Not All That Glitters Is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018
9/23/2018 New Drug Update: Not All That Glitters is Gold Idaho Society of Health‐System Pharmacists 2018 Fall Meeting Sun Valley, Idaho September 30, 2018 PRESENTERS: Boise VA Medical Center Idaho State University PGY2 Ambulatory Care Residents: PGY2 Pharmacotherapy Resident: Audra Wilson, PharmD; Kat Liu, PharmD; Kailee Morton, PharmD Nitz Bankova, PharmD St. Luke’s Health System Infectious Disease Pharmacy Fellow: PGY2 Oncology Pharmacy Residents: Benjamin Pontefract, PharmD Andrew Li, PharmD; Amanda Wright, PharmD; Bryce Benson, PharmD PGY2 Mental Health Pharmacy Resident: Samantha Patton, PharmD Disclosures No conflicts of interest to disclose Learning Objectives • Describe clinical scenarios where newly approved medications are beneficial. • Compare newly approved medications with current standards of care. • Identify investigational new drugs and their potential clinical applications. 1 9/23/2018 Overview of Topics • Infectious Disease • Ambulatory Care • Diabetes Mellitus • Women’s Health • Anticoagulation • Pain Management • Oncology • Acute Myeloid Leukemia (AML) Infectious Disease: Plazomicin Delafloxacin Benjamin Pontefract, PharmD Plazomicin (Zemdri®):Overview • Systemic antibiotic in the aminoglycoside family • MoA: disrupts the 30s ribosome to prevent protein synthesis • Concentration dependent bactericidal activity • Approved to treat UTIs caused by E. coli, K. pneumoniae, Proteus spp, and Enterobacter cloaecae 2 9/23/2018 Aminoglycoside Shortcomings Toxicities Resistance • Nephrotoxicity • Decreased cell permeability • Ototoxicity • Altered ribosome binding sites • Therapeutic drug monitoring • AMG‐modifying enzymes (AME) Plazomicin: Efficacy • Study 009: • RCT comparing plazomicin IV to meropenem IV for cUTI caused by enterobactereceae • Plazomicin group was non‐inferior to meropenem group • Study 007: • RCT comparing plazomicin IV to polymyxin E IV for BSI cause by carbapenem‐resistant Enterobacteriaceae (CRE) • Plazomicin was associated with less mortality compared to polymyxin E Connolly L, Achaogen, Inc. -

Denosumab, Raloxifene, Romosozumab and Teriparatide to Prevent Osteoporotic Fragility Fractures: a Systematic Review and Economic Evaluation
Journals Library Health Technology Assessment Volume 24 • Issue 29 • June 2020 ISSN 1366-5278 Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation Sarah Davis, Emma Simpson, Jean Hamilton, Marrissa Martyn-St James, Andrew Rawdin, Ruth Wong, Edward Goka, Neil Gittoes and Peter Selby DOI 10.3310/hta24290 Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation Sarah Davis ,1* Emma Simpson ,1 Jean Hamilton ,1 Marrissa Martyn-St James ,1 Andrew Rawdin ,1 Ruth Wong ,1 Edward Goka ,1 Neil Gittoes 2 and Peter Selby 3 1Health Economics and Decision Science, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK 2University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK 3School of Medical Sciences, University of Manchester, Manchester, UK *Corresponding author Declared competing interests of authors: Neil Gittoes reports personal fees for being a member of the advisory board to Union Chimique Belge (UCB) S.A. (Brussels, Belgium) and personal fees for contributing to educational meeting sponsored by Eli Lilly and Company (Indianapolis, IN, USA), outside the submitted work. He is also a trustee of the National Osteoporosis Society, a member of the advisory board of the National Osteoporosis Guideline Group and Deputy Chairperson of the Specialised Endocrinology Clinical Reference Group, NHS England. Published June 2020 DOI: 10.3310/hta24290 This report should be referenced as follows: Davis S, Simpson E, Hamilton J, James MM-S, Rawdin A, Wong R, et al. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. -

NCT04179474 Study ID: 3110‐108‐002 Title: A
NCT04179474 Study ID: 3110‐108‐002 Title: A Phase 1b, Two‐Part, Open‐Label, Fixed‐Sequence, Safety, Tolerability and Drug‐Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Date: 14Aug2019 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Title Page Protocol Title: A Phase 1b, Two-Part, Open-Label, Fixed-Sequence, Safety, Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Number: 3110-108-002 Product: Ubrogepant (AGN-241688, MK-1602) Brief Protocol Title: Drug-Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab Study Phase: 1b Sponsor Name and Legal Registered Address: Allergan Pharmaceuticals International Limited, Clonshaugh Industrial Estate, Coolock, Dublin 17, Ireland US Agent: Allergan Sales, LLC, 2525 Dupont Dr, Irvine, California 92612, USA Regulatory Agency Identifying Number(s): IND #113924 SAE Reporting Fax Number/Email: Business Unit Email Phone Fax Allergan Approval Date: 14 August 2019 1 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Sponsor Signatories: Date Date The signatures of the sponsor signatories are collected on the protocol approval page. 2 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Table of Contents Title Page .........................................................................................................................................1 -
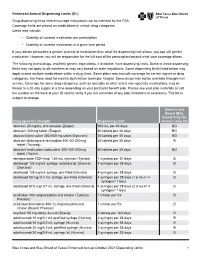
Enhanced Annual Drug List Dispensing Limits
Enhanced Annual Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral solution (Ziagen) 960 mL per 30 days BG abacavir 300 -

761063Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761063Orig1s000 CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S) Office of Clinical Pharmacology Review BLA Number 761063 Link to EDR \\cdsesub1\evsprod\bla761063\0001\m1\us\cover.pdf Submission Date 10/27/2017 Submission Type NME, Standard Review Brand Name EMGALITYTM Generic Name Galcanezumab Dosage Form and Strength 120 mg/mL Prefilled auto-injector pen and Pre-filled syringe Route of Administration Subcutaneous injection Proposed Dose/Regimen: 120 mg once monthly, with a 240 mg loading dose as the initial dose Proposed Indication preventive treatment of migraine Applicant Eli Lilly and Company Associated IND 111295 OCP Review Team Bilal AbuAsal, Ph.D., Gopichand Gottipati, Ph.D., Kevin Krudys, Ph.D., Sreedharan Sabarinath, Ph.D. OCP Final Signatory Ramana Uppoor, Ph.D. 1 Reference ID: 4278873 Table of Contents 1. EXECUTIVE SUMMARY .......................................................................................................................... 3 1.1 Recommendations ........................................................................................................................ 3 1.2 Post-Marketing Requirements and Commitments ....................................................................... 4 2. SUMMARY OF CLINICAL PHARMACOLOGY ASSESSMENT ..................................................................... 5 2.1 Pharmacology and Clinical Pharmacokinetics............................................................................... 5 2.2 Dosing and -
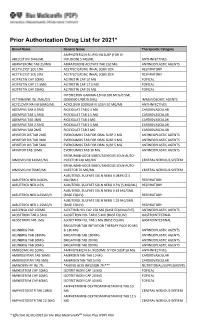
Prior Authorization Drug List for 2021*
Prior Authorization Drug List for 2021* Brand Name Generic Name Therapeutic Category AMPHOTERICIN B LIPID INJ SUSP (FOR IV ABELCET INJ 5MG/ML INFUSION) 5 MG/ML ANTI-INFECTIVES ABIRATERONE TAB 250MG ABIRATERONE ACETATE TAB 250 MG ANTINEOPLASTIC AGENTS ACETYLCYST SOL 10% ACETYLCYSTEINE INHAL SOLN 10% RESPIRATORY ACETYLCYST SOL 20% ACETYLCYSTEINE INHAL SOLN 20% RESPIRATORY ACITRETIN CAP 10MG ACITRETIN CAP 10 MG TOPICAL ACITRETIN CAP 17.5MG ACITRETIN CAP 17.5 MG TOPICAL ACITRETIN CAP 25MG ACITRETIN CAP 25 MG TOPICAL INTERFERON GAMMA-1B INJ 100 MCG/0.5ML ACTIMMUNE INJ 2MU/0.5 (2000000 UNIT/0.5ML) IMMUNOLOGIC AGENTS ACYCLOVIR NA INJ 50MG/ML ACYCLOVIR SODIUM IV SOLN 50 MG/ML ANTI-INFECTIVES ADEMPAS TAB 0.5MG RIOCIGUAT TAB 0.5 MG CARDIOVASCULAR ADEMPAS TAB 1.5MG RIOCIGUAT TAB 1.5 MG CARDIOVASCULAR ADEMPAS TAB 1MG RIOCIGUAT TAB 1 MG CARDIOVASCULAR ADEMPAS TAB 2.5MG RIOCIGUAT TAB 2.5 MG CARDIOVASCULAR ADEMPAS TAB 2MG RIOCIGUAT TAB 2 MG CARDIOVASCULAR AFINITOR DIS TAB 2MG EVEROLIMUS TAB FOR ORAL SUSP 2 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 3MG EVEROLIMUS TAB FOR ORAL SUSP 3 MG ANTINEOPLASTIC AGENTS AFINITOR DIS TAB 5MG EVEROLIMUS TAB FOR ORAL SUSP 5 MG ANTINEOPLASTIC AGENTS AFINITOR TAB 10MG EVEROLIMUS TAB 10 MG ANTINEOPLASTIC AGENTS ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 140MG/ML INJECTOR 140 MG/ML CENTRAL NERVOUS SYSTEM ERENUMAB-AOOE SUBCUTANEOUS SOLN AUTO- AIMOVIG INJ 70MG/ML INJECTOR 70 MG/ML CENTRAL NERVOUS SYSTEM ALBUTEROL SULFATE SOLN NEBU 0.083% (2.5 ALBUTEROL NEB 0.083% MG/3ML) RESPIRATORY ALBUTEROL NEB 0.5% ALBUTEROL SULFATE -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery.