Dissolution Kinetics of Secondary Covellite Resulted from Digenite Dissolution in Ferric/Acid/Chloride Media
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

PHASE CHANGES in Cu 2 S AS a FUNCTION of TEMPERATURE 1. PREVIOUS WORK
National Bureau of Standards Special Publication 364, Solid State Chemistry, Proceedings of 5th Materials Research Symposium, issued July 1972. PHASE CHANGES IN cu2s AS A FUNCTION OF TEMPERATURE William R. Cook, Jr. Gould Inc., Gould Laboratories Cleveland, Ohio 44108 and Case Western Reserve University Cleveland, Ohio 44106 The high-'copper phase boundary of Cu2s deviates from stoichiometry above 300 °C, first becoming copper deficient, then above - 1075 °C becoming copper rich. The maximum copper content occurs at the monotectic temperature of 1104 °C. The strong effect of oxygen on the hexagonal-cubic transition in Cu2S was confirmed; the transition was also found to be sensi tive to the type of pretreatment of the material. The high temperature tetragonal "Cu1 96s" phase is stable between Cu1.95S and Cu2s, at temperatures of - 90° to - 140 °C. The tr~nsi tion to the tetragonal phase is extremely sluggish. The true composition of djurleite has been shown to be approximately Cu1.93S. The phases near the chalcocite-digenite region of the diagram may be grouped into those with hexagonal close packing of sulfur atoms and those with cubic close packing of sulfurs. This is important in understanding rates of transformation among the various phases that occur in this area of the diagram. Key words: Chalcocite; cu2s; digenite; djurleite; nonstoichiometry; phase relations. 1. PREVIOUS WORK Fairly thorough explorations of the Cu-s phase diagram between cu2s and cu1• ,shave been made by a number of previous workers [1-8] 1 The resulting diagram may be seen in figure 1 taken largely from Roseboom [7] and Riu [8]. -
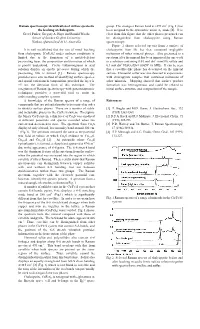
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao Del Norte, Philippines
Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao del Norte, Philippines by Allan Maglaya Ignacio B.Sc. Geology, National Institute of Geological Sciences University of the Philippines Thesis submitted in partial fulfilment of the requirements of the Masters of Economic Geology Degree Centre for Ore Deposit Research, University of Tasmania December, 2005 DECLARATION OF ORIGINALITY This thesis contains no material which has been accepted for a degree of diploma by the University of Tasmania or any other institution, except by way of background information and duly acknowledged in the thesis, and contains no previous material previously pub- lished or written by another person except where due acknowledgement is given. Allan Maglaya Ignacio 01 December 2005 _________________________ STATEMENT OF AUTHORITY OF ACCESS This thesis may not to be made available for loan or copying for 1.5 years following the date this statement was signed. Following that time, the thesis may be available for loan and lim- ited copying in accordance with Copyright Act 1968. Allan Maglaya Ignacio 01 December 2005 _________________________ TABLE OF CONTENTS Page (s) LIST OF FIGURES …………………………………………………….. i - iii LIST OF APPENDICES ………………………………………………… iv ACKNOWLEDGMENTS ………………………………………………. v ABSTRACT ……………………………………………………………... vi - vii 1.0 INTRODUCTION ………………………………………………………. 1 - 8 1.1 Introduction …………………………………………………………. 1 1.2 Aims and Objectives ……………………………………………….. 1 1.3 Methods Employed …………………………………………………. 2 1.4 Location and Accessibility …………………………………………. 3 1.5 Climate ……………………………………………………………... 5 1.6 Previous Work ……………………………………………………… 5 2.0 GEOLOGICAL SETTING ………………………………………………. 9 - 37 2.1 Introduction ………………………………………………………. 9 2.2 Regional Tectonics …………….…………………………………. 9 2.3 Regional and Local Stratigraphy ………………………………... 11 2.3.1 Basement (Cretaceous-Paleogene) ………………………. 11 2.3.2 Bacuag Formation (Oliogocene-Miocene) .…………….. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

MENDELSOHN (F.), Editor. the Geology of the Northern Rhodesian Copper- Belt
996 BOOK REVIEWS instance 'pits', ' anisotropy', ' cellular texture', ' native iron', are fol- lowed by a 'text-card' with a more detailed description of the sections concerned in English and German. The price is DM 0.75 per photo-card, DM 0.45 per text-card. These prices are reduced by 10% for University departments and academic staff, by 15% for students. The great value of this unique publication is self-evident and does not need further appraisal. Ore-microscopists will welcome the card index as a high-quality source of reference, and university staff will find it a great help in teaching. Economic geologists and nfineralogists will appreciate the straightforward and clear approach, which provides an excellent introduction for research workers less well acquainted with the subject matter. Ore dressing workers and metallurgists will be able to Use the card index for the solution of their problems without having to go into details of ore mineralogy. The authors should be warmly con- gratulated on this first part of a very fine piece of work. A. F. H. MENDELSOHN (F.), editor. The Geology of the Northern Rhodesian Copper- belt. London (Macdonald & Co. Ltd.), 1961, xvi+523 pp., 185 figs. Price 8r Twenty-three authors have contributed to this comprehensive volume. Part 1 provides a general account of the physiography, stratigraphy, structure, metamorphism, and ore deposits of the Copperbelt together with a history of mineral exploration and an account of the methods employed. Part 2 gives detailed descriptions of the individual deposits. In addition to synthesizing the considerable volume of published work on the subject the authors draw extensively on material from unpublished company reports. -

Silver-Rich Central Idaho
Silver-rich Disseminated Sulfides From a Tungsten-bearing Quartz Lode Big Creek District Central Idaho GEOLOGICAL SURVEY PROFESSIONAL PAPER 594-C Silver-rich Disseminated Sulfides From a Tungsten-bearing Quartz Lode Big Creek District Central Idaho By B. F. LEONARD, CYNTHIA W. MEAD, and NANCY CONKLIN SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 594-C Study of a low-grade tungsten deposit whose associated suljide minerals, extracted as waste, are rich in silver and contain some gold UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1968 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 35 cents (paper cover) CONTENTS Page Page Abstract __________________________________________ _ C1 Mineralogy and paragenetic sequence-Continued Introduction ______________________________________ _ 1 Alteration products ____________________________ _ C15 Location and operation _____________________________ _ 4 Paragenetic sequence ___________________________ _ 16 Geology __________________________________________ _ 4 Geologic thermometry __________________________ _ 18 Ore deposit _______________________________________ _ 5 Classification and origin of hypogene mineralization ____ _ 19 Mineralogy and paragenetic sequence ________________ _ 6 Oxidation and enrichment ___ ------------------------ 20 Typical ore ___________________________________ _ 6 Economic considerations ____________________________ _ 21 Gangue indicated by mill products _______________ _ 7 Acknowledgments ___________________ .:. ______________ _ 23 Tungsten minerals _______ ,______________________ _ 8 References ________________________________________ _ 23 Sulfides and related minerals ____________________ _ 8 ILLUSTRATIONS [Plates follow page C24f - PLATE 1. Drawing and X-ray micrographs of acanthite and copper sulfides on galena. 2. Drawing and X-ray micrographs of electrum in pyrite. 3. -

Covellite) Probed by NQR
Phase transition and anomalous electronic behavior in layered dichalcogenide CuS (covellite) probed by NQR R.R. Gainov 1*, A.V. Dooglav 1, I.N. Pen’kov 2, I.R. Mukhamedshin 1,3, N.N. Mozgova 4, A.V. Evlampiev 1, and I.A. Bryzgalov 5 1 Department of Physics, Magnetic RadioSpectroscopy Laboratory, Kazan State University, Kazan, Kremlevskaya str. 18, 420008, Russian Federation 2 Department of Geology, Kazan State University, Kazan, Kremlevskaya str. 4/5, 420111, Russian Federation 3 Laboratoire de Physique des Solides, UMR 8502, Universite Paris-Sud, 91405 Orsay, France 4 Institute of geology of ore deposits, petrography, mineralogy and geochemistry (Russian Academy of Science), Staromonetny per. 35, Moscow 109017, Russian Federation 5 Department of Geology, Moscow State University, Moscow, Vorob’evy gory, 119991, Russian Federation * Corresponding author: tel.: 7-843-2315175; fax.: 7-843-2387201. Electronic address: [email protected] (R.R. Gainov). Nuclear quadrupole resonance (NQR) on copper nuclei has been applied for studies of the electronic properties of quasi-two-dimensional low-temperature superconductor CuS (covellite) in the temperature region between 1.47 and 290 K. Two NQR signals corresponding to two non- equivalent sites of copper in the structure, Cu(1) and Cu(2), has been found. The temperature dependences of copper quadrupole frequencies, line-widths and spin-lattice relaxation rates, which so far had never been investigated so precisely for this material, altogether demonstrate the structural phase transition near 55 K, which accompanies transformations of electronic spectrum not typical for simple metals. The analysis of NQR results and their comparison with literature data show that the valence of copper ions at both sites is intermediate in character between monovalent and divalent states with the dominant of the former. -

Mineralogy, Textures, and Relative Age Relationships of Massive Sulfide Ore in the West Shasta District, California
Econom/cGeo/og• Vol. 80, 1985, pp. 2114-2127 Mineralogy, Textures, and Relative Age Relationshipsof MassiveSulfide Ore in the West ShastaDistrict, California STEPHEN S. HOWE U.S. GeologicalSurvey, 345 MiddlefieldRoad, Mail Stop901, Menlo Park, California 94025 Abstract The Devonian massive sulfide orebodies of the West Shasta district in northern California are composedprimarily of pyrite, with lesseramounts of other sulfideand gangueminerals. Examinationof polishedthin sectionsof more than 100 samplesfrom the Mammoth,Shasta King, Early Bird, Balaklala,Keystone, and Iron Mountainmines suggests that mineralization may be dividedinto six parageneticstages, the last five each separatedby an episodeof deformation:(1) precipitationof fine-grained,locally colloformand framboidalpyrite and sphalerite;(2) depositionof fine-grainedarsenopyrite and coarse-grainedpyrite, the latter enclosingtiny inclusionsof pyrrhotite;(3) penetrationand local replacement of sulfideminerals of stages1 and 2 alonggrowth zones and fracturesby chalcopyrite,sphalerite, galena, ten- nantite, pyrrhotite, bornite, and idaite; (4) recrystallizationand remobilizationof existing minerals,locally increasingtheir size and euhedralismand promotingtheir aggregation;(5) depositionof quartz, white mica, chlorite, and calcite;and (6) formationof bornite, digenite, chalcocite,and covellite during supergene enrichment of severalorebodies at the Iron Mountain mine.Despite regional greenschist facies metamorphism and local heating by intrusivebodies, enoughof the originaldepositional -

149. Crystal Structure of Enargite (Cu3ass4)
524 [Vol. 9, 149. Crystal Structure of Enargite (Cu3AsS4). By Katsutoshi TAKANE. Institute of Mineralogy, Petrology and Economic Geology, Tohoku Imperial University, Sendai. (Rec. Nov. 11, 1933. Comm. by S. Kozu, M.I.A., Nov. 13, 1933.) Recently, the crystal structures of copper sulphides such as covellite (CuS), wolfsbergite (CuSbS2), emplectite (CuBiS2), chalcopyrite (CuFeS2) and sulvanite (Cu3VS4), have been worked out by different authors. Among these minerals, sulvanite has been grouped in the mineral family to which enargite belongs, because of the similarity in their chemical compositions. However they are different in crystallographic nature, as sulvanite belongs the cubic system of the space group T1d, determined by Pauling and Hultgren, and enargite belongs to the orthor hombic system of the space group V12h,determined by the present author. Symmetry:-According to the morphological studies already made, enargite belongs to the orthorhombic holodedral class, the axial ratio being given as a : b : c=0.8694 : 1 : 0.8308 by Groth and Mieleitner. The Laue photograph taken from (001) shows no objection to taking the crystal as possessing the symmetry of the orthorhombic holodedral class. It is noteworthy that the photograph indicates a pseudohexagonal symmetry, of which a brief discussion has already been written in Japanese. Unit cell:-From three reflection photographs taken by rotation of three mineral rods parallel to [001], [010] and [100] respectively, immersing in the beam of the CuK ray, the distances of the layer lines were measured, and the results are The axial ratio obtained from the above figures is a : b : c=1.7341.7 1.674, which are double the values of a and c given by the goniometric method. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

USGS Open-File Report 03-107, V. 1.3, Database Record Pages
DepositID 1 Cont AF NameDeposit Mehirize OtherNames Includes Country Code ALGR Algeria StateProvince Lat.Deg 33 Long.Deg 0 Dec.Lat 33.8472222 Lat.Min 50 Long.Min -20 Dec.Long -.341666667 Lat.Sec 50 Long.Sec -30 OreMmt CuGrade% CoGrade% AgGradeppm CuMmt Subtype Redbed Cu Age Cretaceous, Lower Aptian Ma 115 Unit HostRocks Green argillite, sandstone HangingwallBeds FootwallRocks Conglomerate Mineralogy Chalcocite, malachite TraceMinerals Comments Reference Caia, J. 1976, Paleogeographical and sedimentological controls of copper, lead, and zinc mineralization in the Lower Cretaceous sandstones of Africa: Economic Geology, V. 71, p. 409-422 DepositID 2 Cont AF NameDeposit Tansrift OtherNames Tamarift Includes Country Code MRCO Morocco StateProvince Lat.Deg 33 Long.Deg -4 Dec.Lat 33 Lat.Min 0 Long.Min -15 Dec.Long -4.25 Lat.Sec 0 Long.Sec 0 OreMmt 1 CuGrade%1.3 CoGrade% AgGradeppm CuMmt .013 Subtype Redbed Cu Age Cretaceous Ma 120 Unit HostRocks Red sandstone HangingwallBeds FootwallRocks Mineralogy TraceMinerals Comments Reference Caia, J. 1976, Paleogeographical and sedimentological controls of copper, lead, and zinc mineralization in the Lower Cretaceous sandstones of Africa: Economic Geology, V. 71, p. 409-422. Habashi F. and Bassyouni, F.A., 1982, Mineral resources of the Arab countries: Quebec, Chemecon Publishing Ltd.,Laval Univ., 2nd Edition.112 p. Kirkham, R.V, Carriere, J.J., Laramee, R.M., and Garson, D.F., 1994, Global distribution of sediment-hosted stratiform copper deposits and occurrences: Geological Survey of Canada Open File 2915b, 256 p. DepositID 3 Cont AF NameDeposit Tiloula OtherNames Includes Country Code ALGR Algeria StateProvince Lat.Deg 32 Long.Deg 0 Dec.Lat 32.85 Lat.Min 51 Long.Min -27 Dec.Long -.45 Lat.Sec 0 Long.Sec 0 OreMmt CuGrade% CoGrade% AgGradeppm CuMmt Subtype Redbed Cu Age Cretaceous, L.Aptian Ma 115 Unit HostRocks Green argillite, sandstone HangingwallBeds FootwallRocks Conglomerate Mineralogy Chalcocite, malachite TraceMinerals Comments Reference Caia, J.