Nobel Laureates and Twentieth-Century Physics Mauro Dardo Index More Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11/03/11 110311 Pisp.Doc Physics in the Interest of Society 1
1 _11/03/11_ 110311 PISp.doc Physics in the Interest of Society Physics in the Interest of Society Richard L. Garwin IBM Fellow Emeritus IBM, Thomas J. Watson Research Center Yorktown Heights, NY 10598 www.fas.org/RLG/ www.garwin.us [email protected] Inaugural Lecture of the Series Physics in the Interest of Society Massachusetts Institute of Technology November 3, 2011 2 _11/03/11_ 110311 PISp.doc Physics in the Interest of Society In preparing for this lecture I was pleased to reflect on outstanding role models over the decades. But I felt like the centipede that had no difficulty in walking until it began to think which leg to put first. Some of these things are easier to do than they are to describe, much less to analyze. Moreover, a lecture in 2011 is totally different from one of 1990, for instance, because of the instant availability of the Web where you can check or supplement anything I say. It really comes down to the comment of one of Elizabeth Taylor later spouses-to-be, when asked whether he was looking forward to his wedding, and replied, “I know what to do, but can I make it interesting?” I’ll just say first that I think almost all Physics is in the interest of society, but I take the term here to mean advising and consulting, rather than university, national lab, or contractor research. I received my B.S. in physics from what is now Case Western Reserve University in Cleveland in 1947 and went to Chicago with my new wife for graduate study in Physics. -
![I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr](https://docslib.b-cdn.net/cover/8589/i-i-rabi-papers-finding-aid-library-of-congress-pdf-rendered-tue-apr-428589.webp)
I. I. Rabi Papers [Finding Aid]. Library of Congress. [PDF Rendered Tue Apr
I. I. Rabi Papers A Finding Aid to the Collection in the Library of Congress Manuscript Division, Library of Congress Washington, D.C. 1992 Revised 2010 March Contact information: http://hdl.loc.gov/loc.mss/mss.contact Additional search options available at: http://hdl.loc.gov/loc.mss/eadmss.ms998009 LC Online Catalog record: http://lccn.loc.gov/mm89076467 Prepared by Joseph Sullivan with the assistance of Kathleen A. Kelly and John R. Monagle Collection Summary Title: I. I. Rabi Papers Span Dates: 1899-1989 Bulk Dates: (bulk 1945-1968) ID No.: MSS76467 Creator: Rabi, I. I. (Isador Isaac), 1898- Extent: 41,500 items ; 105 cartons plus 1 oversize plus 4 classified ; 42 linear feet Language: Collection material in English Location: Manuscript Division, Library of Congress, Washington, D.C. Summary: Physicist and educator. The collection documents Rabi's research in physics, particularly in the fields of radar and nuclear energy, leading to the development of lasers, atomic clocks, and magnetic resonance imaging (MRI) and to his 1944 Nobel Prize in physics; his work as a consultant to the atomic bomb project at Los Alamos Scientific Laboratory and as an advisor on science policy to the United States government, the United Nations, and the North Atlantic Treaty Organization during and after World War II; and his studies, research, and professorships in physics chiefly at Columbia University and also at Massachusetts Institute of Technology. Selected Search Terms The following terms have been used to index the description of this collection in the Library's online catalog. They are grouped by name of person or organization, by subject or location, and by occupation and listed alphabetically therein. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Heisenberg's Visit to Niels Bohr in 1941 and the Bohr Letters
Klaus Gottstein Max-Planck-Institut für Physik (Werner-Heisenberg-Institut) Föhringer Ring 6 D-80805 Munich, Germany 26 February, 2002 New insights? Heisenberg’s visit to Niels Bohr in 1941 and the Bohr letters1 The documents recently released by the Niels Bohr Archive do not, in an unambiguous way, solve the enigma of what happened during the critical brief discussion between Bohr and Heisenberg in 1941 which so upset Bohr and made Heisenberg so desperate. But they are interesting, they show what Bohr remembered 15 years later. What Heisenberg remembered was already described by him in his memoirs “Der Teil und das Ganze”. The two descriptions are complementary, they are not incompatible. The two famous physicists, as Hans Bethe called it recently, just talked past each other, starting from different assumptions. They did not finish their conversation. Bohr broke it off before Heisenberg had a chance to complete his intended mission. Heisenberg and Bohr had not seen each other since the beginning of the war in 1939. In the meantime, Heisenberg and some other German physicists had been drafted by Army Ordnance to explore the feasibility of a nuclear bomb which, after the discovery of fission and of the chain reaction, could not be ruled out. How real was this theoretical possibility? By 1941 Heisenberg, after two years of intense theoretical and experimental investigations by the drafted group known as the “Uranium Club”, had reached the conclusion that the construction of a nuclear bomb would be feasible in principle, but technically and economically very difficult. He knew in principle how it could be done, by Uranium isotope separation or by Plutonium production in reactors, but both ways would take many years and would be beyond the means of Germany in time of war, and probably also beyond the means of Germany’s adversaries. -

Communications-Mathematics and Applied Mathematics/Download/8110
A Mathematician's Journey to the Edge of the Universe "The only true wisdom is in knowing you know nothing." ― Socrates Manjunath.R #16/1, 8th Main Road, Shivanagar, Rajajinagar, Bangalore560010, Karnataka, India *Corresponding Author Email: [email protected] *Website: http://www.myw3schools.com/ A Mathematician's Journey to the Edge of the Universe What’s the Ultimate Question? Since the dawn of the history of science from Copernicus (who took the details of Ptolemy, and found a way to look at the same construction from a slightly different perspective and discover that the Earth is not the center of the universe) and Galileo to the present, we (a hoard of talking monkeys who's consciousness is from a collection of connected neurons − hammering away on typewriters and by pure chance eventually ranging the values for the (fundamental) numbers that would allow the development of any form of intelligent life) have gazed at the stars and attempted to chart the heavens and still discovering the fundamental laws of nature often get asked: What is Dark Matter? ... What is Dark Energy? ... What Came Before the Big Bang? ... What's Inside a Black Hole? ... Will the universe continue expanding? Will it just stop or even begin to contract? Are We Alone? Beginning at Stonehenge and ending with the current crisis in String Theory, the story of this eternal question to uncover the mysteries of the universe describes a narrative that includes some of the greatest discoveries of all time and leading personalities, including Aristotle, Johannes Kepler, and Isaac Newton, and the rise to the modern era of Einstein, Eddington, and Hawking. -
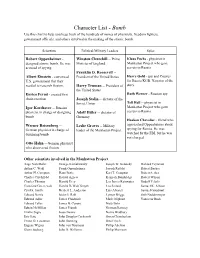
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Commentary & Notes Hans Bethe
J. Natn.Sci.Foundation Sri Lanka 2005 33(1): 57-58 COMMENTARY & NOTES HANS BETHE (1906-2005) Hans Albrecht Bethe who died on 6 March 2005 within the sun is hydrogen which is available in at the age of 98, was one of the most influential tremendous volume. Near the centre of the sun, and innovative theoretical physicists of our time. hydrogen nuclei can be squeezed under enormous pressure to become the element helium. If the Bethe was born in Strasbourg, Alsace- mass of hydrogen nucleus can be written as 1, Lorraine (then part of Germany) on 2 July 1906. Bethe showed that each time four nuclei of His father was a University physiologist and his hydrogen fused together as helium, their sum was mother Jewish, the daughter of a Strasbourg not equal to 4. The helium nucleus weighs about professor of Medicine. Bethe moved to Kiel and 0.7% less, or just 3.993 units of weight. It is this Frankfurt from where he went to Munich to carry missing 0.7% that comes out as a burst of explosive out research in theoretical physics under Arnold energy. The sun is so powerful that it pumps 4 Sommerfeld, who was not only a great teacher, million tons of hydrogen into pure energy every but an inspiring research supervisor as well. second. Einstein's famous equation, E = mc2, Bethe received his doctorate in 1928, for the work provides a clue to the massive energy that reaches he did on electron diffraction. He then went to the earth from the sun, when 4 million tons of the Cavendish laboratory in Cambridge on a hydrogen are multiplied by the figure c2 (square Rockefeller Fellowship, and Rome, before of the speed of light). -

Nuclear Proliferation International History Project
Nuclear Proliferation International History Project Political Authority or Atomic Celebrity? The Influence of J. Robert Oppenheimer on American Nuclear Policy after the Second World War By Marco Borghi NPIHP Working Paper #14 August 2019 THE NUCLEAR PROLIFERATION INTERNATIONAL HISTORY PROJECT WORKING PAPER SERIES Christian F. Ostermann and Leopoldo Nuti, Series Editors This paper is one of a series of Working Papers published by the Nuclear Proliferation International History Project. The Nuclear Proliferation International History Project (NPIHP) is a global network of individuals and institutions engaged in the study of international nuclear history through archival documents, oral history interviews and other empirical sources. Recognizing that today’s toughest nuclear challenges have deep roots in the past, NPIHP seeks to transcend the East vs. West paradigm to work towards an integrated international history of nuclear weapon proliferation. The continued proliferation of nuclear weapons is one of the most pressing security issues of our time, yet the empirically-based study of international nuclear history remains in its infancy. NPIHP’s programs to address this central issue include: • the annual Nuclear Boot Camp for M.A. and Ph.D. candidates to foster a new generation of experts on the international history of nuclear weapons; • the NPIHP Fellowship Program for advanced Ph.D. students and post-doctoral researchers hosted by NPIHP partner institutions around the world; • a coordinated, global research effort which combines archival mining and oral history interviews conducted by NPIHP partners; • a massive translation and digitization project aimed at making documentary evidence on international nuclear history broadly accessible online; • a series of conferences, workshops and seminars hosted by NPIHP partners around the world. -

Curriculum Vitae Vicky (Vassiliki) Kalogera
Curriculum Vitae Vicky (Vassiliki) Kalogera Northwestern University E-mail : [email protected] Phone : (847) 491-5669 Dept of Physics & Astronomy Fax : (847) 467-0679 Address : Technological Institute F234, CIERA - Center for Interdisciplinary 2145 Sheridan Rd., Exploration and Research in Astrophysics Evanston, IL 60208 EDUCATION 1992 { 1997 Ph.D. in Astronomy, University of Illinois at Urbana-Champaign Ph.D. Thesis: \Formation of Low-Mass X-Ray Binaries" Advisor: Prof. Ronald F. Webbink (Univ. of Illinois) 1988 { 1992 Ptihio (B.S.) in Physics, University of Thessaloniki, Greece Diploma Thesis: \Investigations of the Intrinsic Properties of Cataclysmic Binaries" Advisors: Profs. Jan van Paradijs (Univ. of Amsterdam) and John H. Seiradakis (Univ. of Thessaloniki) RESEARCH INTERESTS Astrophysics of Compact Objects (White Dwarfs, Neutron Stars, and Black Holes) Populations of Compact Objects in Binaries and Massive Stars, as Gravitational-Wave Sources, X-ray Binaries, Binary Pulsars, Gamma-Ray Bursts, Supernovae and Supernova Progenitors Formation and Evolution of Binary Systems with Compact Objects in the Milky Way and other galaxies, in Fields and Dense Stellar Environments Time-Domain, Gravitational-Wave, and Transient Astrophysics Advanced Data Analysis and Inference Methods EMPLOYMENT 2017 { Daniel I. Linzer Distinguished University Professor and Professor of Physics and Astronomy 2012 { Director, Center for Interdisciplinary Exploration and Research in Astrophysics (CIERA), Northwestern Univ. 2009 { 2017 E. O. Haven Professor -

Feynman's War: Modelling Weapons, Modelling Nature Peter Galison*
Stud. Hist. Phil. Mod. Phys., Vol. 29, No. 3, 391Ð434, 1998 ( 1998 Published by Elsevier Science Ltd. All rights reserved Printed in Great Britain 1355-2198/98 $19.00#0.00 Feynman’s War: Modelling Weapons, Modelling Nature Peter Galison* What do I mean by understanding? Nothing deep or accurateÐjust to be able to see some of the qualitative consequences of the equations by some method other than solving them in detail. Feynman to Welton, 10 February 1947. 1. Introduction: Modular Culture In all of modern physics there are few elements of theory so pervasive as Feynman diagramsÐJulian Schwinger once sardonically remarked that these simple spacetime figures brought quantum field theory to the masses. In reaching ‘the masses’ this graphic synthesis of quantum mechanics and relativity played a formative role in the development of quantum electrodynamics, meson theory, S-matrix theory, condensed matter physics, the standard model of particle interac- tions, and more recently, string theory. Part representation, part abstract symbol- ism, these line diagrams constitute one of the most intriguing and precise forms of modelling a¤orded by the physical sciences. With stakes that high, it is not surprising that historians, foremost among them Silvan Schweber, have both followed the growth of quantum electrodynamics after the war in detail and precisely tracked antecedents of the Feynman diagrams from cloud chamber photographs to schematic electronic diagrams; from E. C. G. Stueckelberg’s spacetime diagram drawn in the 1930s showing a positron as an electron moving backwards in time to John Wheeler’s emphasis to his student Feynman that all physics could be understood through scattering.1 Once the graphs come * Department of History of Science, Department of Physics, Harvard University, Cambridge, MA 02138, U.S.A. -

The Beginning of the Nuclear Age
The Beginning of the Nuclear Age M. SHIFMAN 1 Theoretical Physics Institute, University of Minnesota 1 Introduction A few years ago I delivered a lecture course for pre-med freshmen students. It was a required calculus-based introductory course, with a huge class of nearly 200. The problem was that the majority of students had a limited exposure to physics, and, what was even worse, low interest in this subject. They had an impression that a physics course was a formal requirement and they would never need physics in their future lives. Besides, by the end of the week they were apparently tired. To remedy this problem I decided that each Friday I would break the standard succession of topics, and tell them of something physics-related but { simultaneously { entertaining. Three or four Friday lectures were devoted to why certain Hollywood movies contradict laws of Nature. After looking through fragments we discussed which particular laws were grossly violated and why. I remember that during one Friday lecture I captivated students with TV sci-fi miniseries on a catastrophic earthquake entitled 10.5, and then we talked about real-life earthquakes. Humans have been recording earthquakes for nearly 4,000 years. The deadliest one happened in China in 1556 A.D. On January 23 of that year, a powerful quake killed an estimated 830,000 people. By today's estimate its Richter scale magnitude was about 8.3. The strongest earthquake ever recorded was the 9.5-magnitude Valdivia earthquake in Chile which occurred in 1960. My remark that in passing from 9.5. -

Copenhagen, a Play by Michael Frayn
© Boldizsar Janko, March 2002 (This is a talk given before the opening show of “Copenhagen”, at The Morris Performing Art Center, South Bend, IN on March 1, 2000) March 1, 2002 I am not aware of any theatrical play in recent memory, or movie, or TV series, for that matter, that is so intensely loaded with scientific content, and nevertheless so incredibly successful as Michael Frayn’s ‘Copenhagen’. The spectacular success of the play in London, New York and Berlin came as a shock to Michael Frayn himself (yes, you guessed it, the author and various directors thought it’s wiser to test things out in smaller cities before bringing the play to Metropolitan South Bend). In March 2000, Michael Frayn was scratching his head and shifting his eyeglasses constantly as he confessed to the following in front of a huge, jam-packed auditorium [quote]: “As I sat in front of my word processor four or five years ago, struggling with this intractable massive material, struggling to understand the outline of the physics involved, struggling to understand the history of the period, struggling to find some shape of what I wanted to say, I lost all faith that the play was ever going to be produced. It seemed to me that I was writing it for my own benefit, that noone was ever going to put on this incredibly abstract remote piece, and even if anyone did, the idea that anyone would come and see it certainly never occured to me.” [end quote]. Well, as his science goes, Mr. Frayn is definitely too modest.