Spatial Patterns of Lepidoptera in the Eucalypt Woodlands of the Sydney Basin, New South Wales, Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
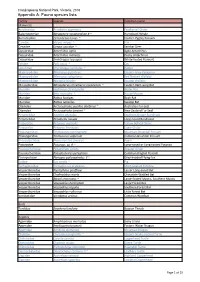
Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -
Home Pre-Fire Moth Species List by Species
Species present before fire - by species Scientific Name Common Name Family Abantiades aphenges Hepialidae Abantiades hyalinatus Mustard Ghost Moth Hepialidae Abantiades labyrinthicus Hepialidae Acanthodela erythrosema Oecophoridae Acantholena siccella Oecophoridae Acatapaustus leucospila Nolidae Achyra affinitalis Cotton Web Spinner Crambidae Aeolochroma mniaria Geometridae Ageletha hemiteles Oecophoridae Aglaosoma variegata Notodontidae Agriophara discobola Depressariidae Agrotis munda Brown Cutworm Noctuidae Alapadna pauropis Erebidae Alophosoma emmelopis Erebidae Amata nigriceps Erebidae Amelora demistis Pointed Cape Moth Geometridae Amelora sp. Cape Moths Geometridae Antasia flavicapitata Geometridae Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela repleta Anthelidae Anthela sp. Anthelidae Anthela varia Variable Anthelid Anthelidae Antipterna sp. Oecophoridae Ardozyga mesochra Gelechiidae Ardozyga sp. Gelechiidae Ardozyga xuthias Gelechiidae Arhodia lasiocamparia Pink Arhodia Geometridae Arrade destituta Erebidae Arrade leucocosmalis Erebidae Asthenoptycha iriodes Tortricidae Asura lydia Erebidae Azelina biplaga Geometridae Barea codrella Oecophoridae Calathusa basicunea Nolidae Calathusa hypotherma Nolidae Capusa graodes Geometridae Capusa sp. Geometridae Carposina sp. Carposinidae Casbia farinalis Geometridae Casbia sp. Geometridae Casbia tanaoctena Geometridae Catacometes phanozona Oecophoridae Catoryctis subparallela Xyloryctidae Cernia amyclaria Geometridae Chaetolopha oxyntis Geometridae Chelepteryx -

The Little Things That Run the City How Do Melbourne’S Green Spaces Support Insect Biodiversity and Promote Ecosystem Health?
The Little Things that Run the City How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Luis Mata, Christopher D. Ives, Georgia E. Garrard, Ascelin Gordon, Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk, Kirsten Parris and Sarah A. Bekessy The Little Things that Run the City – How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Report prepared for the City of Melbourne, November 2015 Coordinating authors Luis Mata Christopher D. Ives Georgia E. Garrard Ascelin Gordon Sarah Bekessy Interdisciplinary Conservation Science Research Group Centre for Urban Research School of Global, Urban and Social Studies RMIT University 124 La Trobe Street Melbourne 3000 Contributing authors Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk and Kirsten Parris. Cover artwork by Kate Cranney ‘Melbourne in a Minute Scavenger’ (Ink and paper on paper, 2015) This artwork is a little tribute to a minute beetle. We found the brown minute scavenger beetle (Corticaria sp.) at so many survey plots for the Little Things that Run the City project that we dubbed the species ‘Old Faithful’. I’ve recreated the map of the City of Melbourne within the beetle’s body. Can you trace the outline of Port Phillip Bay? Can you recognise the shape of your suburb? Next time you’re walking in a park or garden in the City of Melbourne, keep a keen eye out for this ubiquitous little beetle. -

Phylogenomics Reveals Major Diversification Rate Shifts in The
bioRxiv preprint doi: https://doi.org/10.1101/517995; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Phylogenomics reveals major diversification rate shifts in the evolution of silk moths and 2 relatives 3 4 Hamilton CA1,2*, St Laurent RA1, Dexter, K1, Kitching IJ3, Breinholt JW1,4, Zwick A5, Timmermans 5 MJTN6, Barber JR7, Kawahara AY1* 6 7 Institutional Affiliations: 8 1Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 USA 9 2Department of Entomology, Plant Pathology, & Nematology, University of Idaho, Moscow, ID 10 83844 USA 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4RAPiD Genomics, 747 SW 2nd Avenue #314, Gainesville, FL 32601. USA 13 5Australian National Insect Collection, CSIRO, Clunies Ross St, Acton, ACT 2601, Canberra, 14 Australia 15 6Department of Natural Sciences, Middlesex University, The Burroughs, London NW4 4BT, UK 16 7Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 17 *Correspondence: [email protected] (CAH) or [email protected] (AYK) 18 19 20 Abstract 21 The silkmoths and their relatives (Bombycoidea) are an ecologically and taxonomically 22 diverse superfamily that includes some of the most charismatic species of all the Lepidoptera. 23 Despite displaying some of the most spectacular forms and ecological traits among insects, 24 relatively little attention has been given to understanding their evolution and the drivers of 25 their diversity. -

Landcorp Denmark East Development Precinct Flora and Fauna Survey
LandCorp Denmark East Development Precinct Flora and Fauna Survey October 2016 Executive summary Introduction Through the Royalties for Regions “Growing our South” initiative, the Shire of Denmark has received funding to provide a second crossing of the Denmark River, to upgrade approximately 6.5 km of local roads and to support the delivery of an industrial estate adjacent to McIntosh Road. GHD Pty Ltd (GHD) was commissioned by LandCorp to undertake a biological assessment of the project survey area. The purpose of the assessment was to identify and describe flora, vegetation and fauna within the survey area. The outcomes of the assessment will be used in the environmental assessment and approvals process and will identify the possible need for, and scope of, further field investigations will inform environmental impact assessment of the road upgrades. The survey area is approximately 68.5 ha in area and includes a broad area of land between Scotsdale Road and the Denmark River and the road reserve and adjacent land along East River Road and McIntosh Road between the Denmark Mt Barker Road and South Western Highway. A 200 m section north and south along the Denmark Mt Barker Road from East River Road was also surveyed. The biological assessment involved a desktop review and three separate field surveys, including a winter flora and fauna survey, spring flora and fauna survey and spring nocturnal fauna survey. Fauna surveys also included the use of movement sensitive cameras in key locations. Key biological aspects The key biological aspects and constraints identified for the survey area are summarised in the following table. -

Special Edition: Moths Interview with Bart Coppens, Guest Speaker at ICBES 2017
INTERNATIONAL ASSOCI ATION OF BUTTERFLY EXHIBITORS AND SUPPL IERS Volume 16 Number 3 MAI– JUNE 2017 Visit us on the web at www.iabes.org Special edition: moths Interview with Bart Coppens, guest speaker at ICBES 2017 Who are you? I’m Bart Coppens (24) from the Netherlands – a fervent breeder of moths and aspiring entomologist. In my home I breed over 50 species of moths (mainly Saturniidae) on yearly basis. My goal is to expand what started out as a hobby into something more scientific. It turns out the life cycle and biology of many Saturni- idae is poorly known or even unrecorded. By importing eggs and cocoons of rare and obscure species and breeding them in cap- tivity I am able to record undescribed larvae, host plants and the life history of several moth species – information that I publish on a scientific level. My ambition is also to gradually get into more difficult subjects such as the taxonomy, morphology and evolution and perhaps even the organic chemistry (in terms of defensive chemicals) of Saturniidae – but for now these subjects are still beyond my le- Bart with Graellsia isabella vel of comprehension, as relatively young person that has not yet completed a formal education. I’d also like to say I have a general passion for all kinds of Lepidoptera, from butterflies to the tiniest species of moths, I truly like all of them. The reason I mention Saturniidae so much is because I have invested most of my time and expertise into this particular family of Lepidoptera, simply because this order of insects is too big to study on a general scale, so I decided to specialise myself a little in the kinds of moths I find the most impressi- ve and fascinating myself – and was already the most familiar with due to my breeding hobby. -

Foliage Insect Diversity in Dry Eucalypt Forests in Eastern Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 136, 2002 17 FOLIAGE INSECT DIVERSITY IN DRY EUCALYPT FORESTS IN EASTERN TASMANIA by H.J. Elliott, R. Bashford, S.J. Jarman and M.G. Neyland (with four tables, one text-figure and two appendices) ELLIOTT, H.]., BASHFORD, R., JARMAN,S.]' & NEYLAND, M.G., 2002 (3l:xii): Foliage insect diversity in dry eucalypt forests in eastern Tasmania. Papers and Proceedings ofthe Royal Society afTasmania 136: 17-34. ISSN 0080-4703. Forestry Tasmania, 79 Melville St., Hobart, Tasmania 7000, Australia. Species numbers and composition of the insect fauna occurring on trees and shrubs were studied in dry eucalypt forests in eastern Tasmania over nine years. In all, 1164 named and putative species representing 17 orders and 157 families were collected. The bulk of the species belonged to the orders Coleoptera (28%), Hymenoptera (25%), Hemiptera (18%), Lepidoptera (14%) and Diptera (10%). Of the species collected, 388 -- about one-third -- were identified at least to genus or species level. These included 21 named species not previously listed in the Tasmanian insect fauna and 90 undescribed species. A list of 22 host plants for 171 insect species was compiled from records of 132 insect species observed feeding during the study and from previous records ofinsect/host plant associations for 39 insect species found on the study plots. Most insects were feeding on eucalypts (127 insect species) and acacias (38 species). The most widely distributed and commonly collected species were several well-known pests ofeucalypts: Gonipterus scutellatus (Coleoptera: Curculionidae), Uraba lugens (Lepidoptera: N octuidae), Amorbus obscuricornis (Hemiptera: Coreidae), Chaetophyes compacta (Hemiptera: Machaerotidae) and Eriococcus coriaceous(Hemiptera: Eriococcidae). -

December 2 0 1 8 Catalogue
N E W S O U T H P U B L I S H I N G JULY – DECEMBER 2018 CATALOGUE S M A L L P U B L I S H E R O F T H E Y E A R 2 0 1 6 and 2 0 1 7 A powerful reflection on the conditions of mania and how it plays out in our culture where the author raises the stakes by telling his own story. Writing and mental illness make excellent bedfellows, for better or worse. The Rapids – creative and courageous – is an extraordinary personal memoir peppered with film and literary criticism, as well as family history. With reflections on artists such as Carrie Fisher, Kanye West, Robert Lowell, Delmore Schwartz, Paul Thomas Anderson and Spalding Gray, Twyford- Moore also looks at the condition in our digital world, where someone’s manic episode can unfold live in real time, watched by millions. His own story, told unflinchingly, is shocking and sometimes blackly comic. Smart, lively and well- researched, The Rapids manages to be both a wild ride and introspective at once, exploring a condition that touches thousands of people, directly or indirectly. ‘ The Rapids is beautifully written: brimming with humour, empathy, pathos and heart. This book is an earnest, generous, and important contribution to ongoing global dialogue around mental health.’ – Maxine Beneba Clarke, author of The Hate Race The Rapids: Ways of ‘ The Rapids is a remarkable book – intelligent, looking at mania empathic and ethical. It offers a complex and astute Sam Twyford-Moore account of mania and depression both as a cultural phenomenon and a personal experience, and is unafraid of looking at difficult and dark emotions and events. -
Moths Postfire to Feb16 2021 by Family
Species present after fire - by family Scientific Name Common Name Taxon Family Name Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela ocellata Eyespot Anthelid Moth Anthelidae Labdia chryselectra Cosmopterigidae Limnaecia sp. Cosmopterigidae Limnaecia camptosema Cosmopterigidae Macrobathra alternatella Cosmopterigidae Macrobathra astrota Cosmopterigidae Macrobathra leucopeda Cosmopterigidae Ptilomacra senex Cossidae Achyra affinitalis Cotton Web Spinner Crambidae Culladia cuneiferellus Crambidae Eudonia aphrodes Crambidae Hednota sp. Crambidae Hednota bivittella Crambidae Hednota pleniferellus Crambidae Heliothela ophideresana Crambidae Hellula hydralis cabbage centre grub Crambidae Hygraula nitens Pond moth Crambidae Metasia capnochroa Crambidae Metasia dicealis Crambidae Metasia liophaea Crambidae Musotima nitidalis Golden-brown Fern Moth Crambidae Musotima ochropteralis Australian maidenhair fern moth Crambidae Nacoleia oncophragma Crambidae Nacoleia rhoeoalis Crambidae Scoparia chiasta Crambidae Scoparia emmetropis Crambidae Scoparia exhibitalis Crambidae Scopariinae Moss-eating Crambid Snout Moths Crambidae Tipanaea patulella White Rush Moth Crambidae Agriophara confertella Depressariidae Eupselia beatella Depressariidae Eupselia carpocapsella Common Eupselia Moth Depressariidae Eutorna tricasis Depressariidae Peritropha oligodrachma Depressariidae Phylomictis sp. Depressariidae Amata nigriceps Erebidae Cyme structa Erebidae Dasypodia cymatodes Northern wattle moth Erebidae Dasypodia selenophora Southern -

On Australian Moths and Butterflies 5 May 2011
'Barcoding blitz' on Australian moths and butterflies 5 May 2011 the planet within the next decade or so," Dr La Salle said. "This will produce strong benefits for entomology, life sciences and biosecurity." He said barcoding has already achieved some interesting successes in, for example, Europe and the US where it is being used to investigate food fraud, such as selling one type of fish as another type of fish. An Emperor Gum Moth, Opodiphthera eucalypti, well According to Atlas of Living Australia Director, camouflaged amongst Eucalyptus foliage on which they feed Image: CSIRO Donald Hobern, many moths and butterflies are of economic and/or environmental importance to Australia. In just 10 weeks a team of Canadian researchers "Using barcoding for rapid species identification will has succeeded in 'barcoding' 28,000 moth and transform how we handle monitoring of biodiversity butterfly specimens - or about 65 per cent of across Australia and how we respond to potential Australia's 10,000 known species - held at pest arrivals at Australian borders," Mr Hobern said. CSIRO's Australian National Insect Collection (ANIC) in Canberra. "Barcoding for rapid species identification is a powerful new tool which will also assist taxonomists Conducted in collaboration with the Atlas of Living in recognising and describing new species." Australia (ALA) as part of the International Barcode of Life (IBoL), the project involved extracting DNA from each specimen to record its unique genetic Provided by CSIRO code and entering the results, together with an image and other details, to the ALA and ANIC databases. ANIC is the first national collection to integrate the new barcoding approach for a major group of insects. -

Australian Natural History
AUSTRALIAN NATURAL HISTORY MARCH 15, 1967 Registered at the General Post Office, Sydnoy, [or transmissoon by po;t as a periodical VoL. 15, No. 9 PRICE, THIRTY CENTS CONTENTS PAGE THE STORY OF THE ROTH ETHNOGRAPHIC COLLECTION-Karh/een Pope and David R. Moore 273 THE SHORE REEFS OF DARWIN-Eii:::abeth C. Pope 278 REVIEW 284 WATER-A D OuR THIRSTY EARTH-R. J. Griffin 285 YE oMous SEA URCHI IN SYD EY HARBOUR-Eii:::aberh C. Pope 289 AUSTRALIA SEALS-B. J. Marlo1r 290 RING-TAILED POSSUMS-Michae/ Marsh 294 LARGE ALP! E STONEFLY 298 AUTHOR'S COMMENT 0 BOOK REVIEW 298 WEBWORM. ( SECT PEST OF THE WHEATLANDS-L. £. Koch 299 THE TASMANIA �MUSEUM-W. Bryden 303 e FRONT COVER: Aboriginal ceremonial regalia on the west coast of Cape York Peninsula, near Mapoon Mission. The carved heads represent crocodiles. There is obvious inHuence from Torres Strait in this type of mask. The photo was taken by W. E. Roth, who was Protector of Aboriginals in orthern Queensland in the late 1890's and the early years of this century. An article on his work and his remarkable collection of Aboriginal artefacts from northern Queensland appears on the opposite page. VoL. 15, o. 9 MARCH 15, 1967 AUS IAN NATUR ISTORY Published Quarterly by the Australian Museum College Street, Sydney Editor: F. H. Talbot, M.Sc., Ph.D. Annual Subscription, Posted, $1 .40 VoL. 15, o. 9 MARCH 15, 1967 THE STORY OF THE ROTH ETHNOGRAPHIC COLLECTION By KATHLEE POPE, Museum Assistant, and DAVID R. MOORE, Curator of Anthropology, Australian Museum 1906 the Australian Museum acquired South Australian School of Mines and I one of the most complete and well lndu trie , and assistant master at Sydney documented collections of ethnographic Grammar S:hool.