Khellactone Derivatives and Other Phenolics of Phlojodicarpus Sibiricus (Apiaceae): HPLC-DAD-ESI-QQQ-MS/MS and HPLC-UV Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poaceae: Aveneae) in Northern Asia
Acta Biol. Univ. Daugavp. 11 (2) 2011 ISSN 1407 - 8953 ON THE ORIGIN OF SOME SPECIES OF THE GENUS AGROSTIS L. (POACEAE: AVENEAE) IN NORTHERN ASIA Ilya Enushchenko Enushchenko I. 2011. On the origin of some species of the genus Agrostis L. (Poaceae: Aveneae) in Northern Asia. Acta Biol. Universit. Daugavpil., 11 (2): 113-118. Critical revision of some species of the genus Agrostis L. (Poaceae: Aveneae) in Northern Asia was carried out. The paper considers the hybridization process within the genus. Suppositions about the kinship and origin of some species are made. New taxonomic rank A. bodaibensis, A. korczaginii, and A. ussuriensis is substantiated. Key words: Agrostis, Poaceae, hybrids, kinship, Northern Asia. Ilya Enushchenko. V.B. Sochava Institute of Geography the Siberian Branch of the Russian Academy of Sciences, Ulan-Bator, 1, 664033, Irkutsk, Russia, [email protected] INTRODUCTION Agraulus (Beauv.) Tzvel., and sect. Trichodium (Michx.) Dumort. The genus Agrostis L. includes about 150 species. It is one of the largest and most Species of the first section are characterized by polymorphic genera of the tribe Aveneae awnless (normally), less frequently aristate lem- Dumort., therefore it is considered taxo- mas. Callus hairs are generally more than 6 times nomically complex. Representatives of shorter than lemmas, more rarely are absent. the genus are distributed almost in all Paleae are 1.5 – 2 times shorter than lemmas. An- extratropical areas of both hemispheres; thers are 0.8 – 1.5 mm long. This section includes they also occur at high altitudes of the predominantly tetraploid (2n=28), more rarely tropics (Tsvelev 1976). -

Structure, Condition, and Prospects of Electrical Grids in the Republic of Sakha (Yakutia)
E3S Web of Conferences 124, 04001 (2019) https://doi.org/10.1051/e3sconf/201912404001 SES-2019 Structure, condition, and prospects of electrical grids in the Republic of Sakha (Yakutia) N. S. Volotkovskaya1, N. N. Kugusheva1,2, A. S. Semenov1,2,*, I. A. Yakushev1,2, S. N. Pavlova1,3, and O. V. Kolosova4 1 North-Eastern Federal University n.a. M.K. Ammosov, Polytechnic Institute (branch) in Mirny, Sakha (Yakutia), Russia 2 North-Eastern Federal University n.a. M.K. Ammosov, Institute of Mathematics and Information Science, Sakha (Yakutia), Russia 3 North-Eastern Federal University n.a. M.K. Ammosov, Institute of Finances and Economics, Sakha (Yakutia), Russia 4 Peter the Great St. Petersburg Polytechnic University, St Petersburg, Russia Abstract. The paper analyzes the condition of electrical grids in the west of the Republic of Sakha (Yakutia); data sampled for the last 10 years. It demonstrates the geographic location of grids, which defines the scale of the study. Technical indicators are presented for 10 years; they reflect an increase in the fixed assets. The paper derives mathematical models of the wear of transmission equipment used in the western grids. It proves that the condition of equipment will deteriorate further unless its maintenance is properly funded. The paper analyzes the prospects of electrical grids in the Republic of Sakha (Yakutia). It presents a program for local energy optimization. The costs associated with five alternative development scenarios are calculated and presented in a tabular format. 1 Introduction 30% of the total heat in the Republic. HPPs account for 37.5% of the installed capacity. -

Buryat Cumhuriyeti'nin Turizm Potansiyeli Ve Başlıca
SDÜ FEN-EDEBİYAT FAKÜLTESİ SOSYAL BİLİMLER DERGİSİ, AĞUSTOS 2021, SAYI: 53, SS. 136-179 SDU FACULTY OF ARTS AND SCIENCES JOURNAL OF SOCIAL SCIENCES, AUGUST 2021, No: 53, PP. 136-179 Makale Geliş | Received : 07.06.2021 Makale Kabul | Accepted : 31.08.2021 Emin ATASOY Bursa Uludağ Üniversitesi, Türkçe ve Sosyal Bilimler Eğitimi Bölümü [email protected] ORCID Numarası|ORCID Numbers: 0000-0002-6073-6461 Erol KAPLUHAN Burdur Mehmet Akif Ersoy Üniversitesi, Coğrafya Bölümü [email protected] ORCID Numarası|ORCID Numbers: 0000-0002-2500-1259 Yerbol PANGALİYEV [email protected] ORCID Numarası|ORCID Numbers: 0000-0002-2392-4180 Buryat Cumhuriyeti’nin Turizm Potansiyeli ve Başlıca Turizm Kaynakları Touristic Potential And Major Touristic Attractions Of Buryatia Republic Öz Rusya Federasyonu’nun Güney Sibirya Bölgesi’nde yer alan Buryat Cumhuriyeti, Saha Cumhuriyeti ve Komi Cumhuriyeti’nden sonra Rusya’nın en büyük yüzölçümüne sahip üçüncü özerk cumhuriyetidir. Siyasi yapılanma olarak Uzakdoğu Federal İdari Bölgesi, ekonomik yapılanma olarak ise Uzakdoğu İktisadi Bölge sınırları içinde yer alan Buryatya, Doğu Sibirya’nın güney kesimlerinde ve Moğolistan’ın kuzeyinde yer almaktadır. Araştırmada coğrafyanın temel araştırma metotları gözetilmiş, kaynak tarama yöntemi aracılığıyla ilgili kaynaklar ve yayınlar temin edilerek veri tabanı oluşturulmuştur. Elde edilen verilerin değerlendirilmesi için haritalar, şekiller ve tablolar oluşturulmuştur. Konunun net anlaşılması amacıyla Buryat Cumhuriyeti’nin lokasyon, Buryat Cumhuriyeti Kültürel Turizm Merkezleri, Buryat Cumhuriyeti’nin Doğal turizm alanları, Buryat Cumhuriyeti milli parkları ve doğa koruma alanları haritalarının yanı sıra ifadeleri güçlendirmek için konular arasındaki bağlantılar tablo ile vurgulanmıştır. Tüm bu coğrafi olumsuzluklara rağmen, Buryatya zengin doğal kaynaklarıyla, geniş Tayga ormanlarıyla, yüzlerce göl ve akarsu havzasıyla, yüzlerce sağlık, kültür ve inanç merkeziyle, çok sayıda kaplıca, müze ve doğa koruma alanıyla, Rusya’nın en zengin turizm kaynaklarına sahip cumhuriyetlerinden biridir. -

Scopoletin 8-Hydroxylase: a Novel Enzyme Involved in Coumarin
bioRxiv preprint doi: https://doi.org/10.1101/197806; this version posted October 4, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron- 2 deficiency responses in Arabidopsis 3 4 Running title: At3g12900 encodes a scopoletin 8-hydroxylase 5 6 Joanna Siwinska1, Kinga Wcisla1, Alexandre Olry2,3, Jeremy Grosjean2,3, Alain Hehn2,3, 7 Frederic Bourgaud2,3, Andrew A. Meharg4, Manus Carey4, Ewa Lojkowska1, Anna 8 Ihnatowicz1,* 9 10 1Intercollegiate Faculty of Biotechnology of University of Gdansk and Medical University of 11 Gdansk, Abrahama 58, 80-307 Gdansk, Poland 12 2Université de Lorraine, UMR 1121 Laboratoire Agronomie et Environnement Nancy- 13 Colmar, 2 avenue de la forêt de Haye 54518 Vandœuvre-lès-Nancy, France; 3INRA, UMR 14 1121 Laboratoire Agronomie et Environnement Nancy-Colmar, 2 avenue de la forêt de Haye 15 54518 Vandœuvre-lès-Nancy, France; 16 4Institute for Global Food Security, Queen’s University Belfast, David Keir Building, Malone 17 Road, Belfast, UK; 18 19 [email protected] 20 [email protected] 21 [email protected] 22 [email protected] 23 [email protected] 24 [email protected] 25 [email protected] 26 [email protected] 27 [email protected] 28 *Correspondence: [email protected], +48 58 523 63 30 29 30 The date of submission: 02.10.2017 31 The number of figures: 9 (Fig. -

Biosynthesis of Daphnetin in Daphne Mezereum L.*
Biosynthesis of Daphnetin in Daphne mezereum L.* Stewart A. Brown Department of Chemistry, Trent University, Peterborough, Ont., Canada K9J 7B8 Z. Naturforsch. 41c, 247—252 (1986); received November 4, 1985 Biosynthesis, Coumarins, Daphne mezereum, Daphnetin Shoots of Daphne mezereum synthesized daphnetin (7,8-dihydroxycoumarin) more efficiently from [2-l4C]umbelliferone (7-hydroxycoumarin) than from [2-l4C]p-coumaric acid, and [2-14C]caf- feic acid was more poorly utilized still. These findings do not support the idea of derivation of daphnetin via hydroxylation of the caffeic acid ring at the 2 position, followed by lactone ring formation; instead they are consistent with the concept of daphnetin formation by an additional hydroxylation of umbelliferone at C-8. Umbelliferone was recovered with little l4C dilution from emulsin-hydrolysed extracts of shoots fed labelled umbelliferone, and TLC of extracts from un treated shoots revealed two substances yielding umbelliferone on hydrolysis. Evidence is pre sented from TLC and HPLC analysis that one of these is skimmin (7-O-ß-D-glucosylumbel- liferone), not previously reported from Daphne. The tracer experiments further support the theory that umbelliferone is the general precursor of coumarins bearing two or more hydroxyl functions on the aromatic ring. Introduction (Thymelaeaceae), a hardy perennial shrub, where it Approximately 70 coumarins with the 7,8 oxygen occurs together with its 7-ß-D-glucoside, daphnin ation pattern' have been reported to be naturally (6b) and daphnetin glucoside (6c), the 8-O-ß-D- occurring [1], over half being furanocoumarins de g lu c o s y la te d is o m e r [1]. -

A Detailed Review on Morphotaxonomy And
Acta Scientific Microbiology (ISSN: 2581-3226) Review Article Volume 1 Issue 6 June 2018 Skimmia anquetilia N.P. Taylor and Airy Shaw A Detailed Review on Morphotaxonomy and Chemoprofiling of Saduf Nissar1, Neelofar Majid1, Aabid M Rather1*, Irshad A Nawchoo1 and GG Mohi-Ud-Din2 1Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, Department of Botany, University of Kashmir, Srinagar, India 2Department of Botany, Government Degree College for Women, Sopore, Baramullah, India *Corresponding Author: Aabid M Rather, Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, DepartmentReceived: April of Botany, 20, 2018; University Published: of Kashmir, May 28, Srinagar, 2018 India. Abstract - In recent times, medicinal plants have attracted huge attention due to their diverse range of biological and therapeutic proper Skimmia ties. Evidences have been accumulated since ages to demonstrate promising potential of medicinal plants used in various traditional, anquetilia complementary, and alternative systems with the ever-increasing interest of today’s population towards natural products, Rutaceae N.P. Taylor and Airy Shaw emerged out to be one of the most eye-catching plant bearing multiple medicinal properties. It is a perennial aromatic evergreen shrub belonging to family . Pharmacological studies have demonstrated significant action - of different extracts as antimicrobial, anti-inflammatory agents, among others, supporting some of its popular uses. An attempt has S. anquetilia been made in this review article to provide an up-to-date overview of the morphological parameters, taxonomic features, distribu tion pattern, traditional uses, as well as the phytochemistry and biological activities of . The present review provides insights for future research aiming for both ethnopharmacological validation of its popular use and its exploration as a new source ofKeywords herbal drugs: Skimmia and/or anquetilia; bioactive Rutaceaenatural products. -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10 -
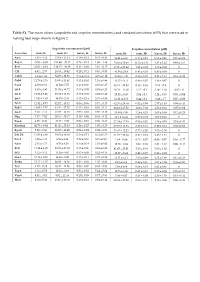
(Scopoletin and Scopolin Concentrations) and Standard Deviations (±SD) That Were Used in Making Heat Maps Shown in Figure 2
Table S1. The mean values (scopoletin and scopolin concentrations) and standard deviations (±SD) that were used in making heat maps shown in Figure 2. Scopoletin concentration [µM] Scopolin concentration [µM] Accession roots, H- roots, H+ leaves, H- leaves, H+ roots, H- roots, H+ leaves, H- leaves, H+ An-1 4.91 ± 4.12 15.19 ± 11.34 0.10 ± 0.12 0.45 ± 0.24 14.81 ± 4.05 0.13 ± 0.12 0.68 ± 0.41 0.05 ± 0.08 Bay-0 41.93 ± 6.58 151.90 ± 30.12 0.29 ± 0.18 1.99 ± 1.46 38.95 ± 10.69 07.15 ± 6.36 3.81 ± 1.22 0.88 ± 1.53 Br-0 35.01 ± 9.48 139.27 ± 49.38 0.31 ± 0.09 2.61 ± 0.27 67.26 ± 23.82 1.22 ± 0.08 1.65 ± 0.08 0 C24 4.92 ± 2.67 16.63 ± 10.42 0.20 ± 0.11 0.83 ± 0.55 41.36 ± 24.8 0.43 ± 0.19 0.85 ± 0.59 0 Can-0 3.14 ± 1.89 64.03 ± 84.91 0.14 ± 0.14 0.37 ± 0.12 34.63 ± 4.85 0.56 ± 0.05 2.23 ± 1.24 0.19 ± 0.33 Col-0 11.79 ± 3.16 29.60 ± 11.41 0.31 ± 0.41 1.16 ± 0.99 11.47 ± 3.41 0.46 ± 0.07 1.48 ± 0.67 0 Cvi-1 4.58 ± 0.62 11.54 ± 7.57 0.19 ± 0.10 0.61 ± 0.27 35.41 ± 32.83 0.35 ± 0.16 2.15 ± 0.8 0 Eil-0 8.03 ± 4.90 21.55 ± 14.72 0.15 ± 0.03 0.53 ± 0.23 28.24 ± 11.10 1.12 ± 0.2 2.19 ± 1.16 0.02 ± 0 Eri-0 12.86 ± 3.96 23.84 ± 13.33 0.13 ± 0.09 0.68 ± 0.48 20.55 ± 8.45 2.04 ± 0.3 2.24 ± 0.93 0.03 ± 0.04 Est-1 14.85 ± 1.63 36.79 ± 2.44 0.42 ± 0.19 2.01 ± 0.86 32.22 ±12.66 0.44 ± 0.2 6.48 ± 3.7 0.07 ± 0.09 Fei-0 12.82 ± 8.73 52.02 ± 15.32 0.09 ± 0.06 0.73 ± 0.37 62.36 ± 28.64 0.52 ± 0.08 2.77 ± 1.53 0.08 ± 0.13 Fuk-1 13.06 ± 7.87 44.36 ± 47.37 0.13 ± 0.01 0.62 ± 0.11 29.23 ± 27.52 8.09 ± 7.02 1.58 ± 0.62 0.05 ± 0.09 Ga-0 3.40 ± 1.11 19.67 ± 12.65 0.03 -

Sovereignty and Territorial Integrity)
FINANCIAL REPORTING AUTHORITY (CAYFIN) Delivery Address: th Mailing Address: 133 Elgin Ave, 4 Floor P.O. Box 1054 Government Administrative Building Grand Cayman KY1-1102 Grand Cayman CAYMAN ISLANDS CAYMAN ISLANDS Direct Tel No. (345) 244-2394 Tel No. (345) 945-6267 Fax No. (345) 945-6268 Email: [email protected] Financial Sanctions Notice 02/10/2020 Ukraine (Sovereignty and Territorial Integrity) Introduction 1. Council Regulation (EU) 269/2014 (“the Regulation”) imposing financial sanctions against those undermining or threatening the sovereignty and territorial integrity of Ukraine has been amended so that an asset freeze now applies to the persons listed in the Annex to this Notice. Notice summary (Full details are provided in the Annex to this Notice) 2. The following entries have been added to the consolidated list and are now subject to an asset freeze. • Alexander Nikolaevich GANOV (Group ID: 13926) • Leonid Kronidovich RYZHENKIN (Group ID: 13927) • JOINT-STOCK COMPANY ‘LENPROMTRANSPROYEKT’ (Group ID: 13928) • JOINT-STOCK COMPANY ‘THE BERKAKIT-TOMMOT-YAKUTSK RAILWAY LINE’S CONSTRUCTION DIRECTORATE’ (Group ID: 13929) • FEDERAL STATE UNITARY ENTERPRISE ‘CRIMEA RAILWAY’ (Group ID: 13930) • FIRST CRIMEAN INSURANCE COMPANY (Group ID: 13931) What you must do 3. You must: i. check whether you maintain any accounts or hold any funds or economic resources for the persons set out in the Annex to this Notice; ii. freeze such accounts, and other funds or economic resources; iii. refrain from dealing with the funds or assets or making them available (directly or indirectly) to such persons unless licensed by the Governor; iv. report any findings to the FRA at [email protected], together with any additional information that would facilitate compliance with the Regulation; v. -

Phenylpropanoids
Phenylpropanoids The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds. Concentrations of phenylpropanoids within plants are also altered by changes in resource availability. www.MedChemExpress.com 1 Phenylpropanoids Inhibitors & Modulators (+)-Columbianetin (+)-Columbianetin acetate ((S)-Columbianetin) Cat. No.: HY-N0363 ((S)-Columbianetin acetate) Cat. No.: HY-N0363A (+)-Columbianetin is an isomer of Columbianetin. (S)-Columbianetin acetate is an isomer of Columbianetin is a phytoalexin associated with Columbianetin. Columbianetin is a phytoalexin celery (Apium graveolens) resistance to associated with celery (Apium graveolens) pathogens during storage. Columbianetin exhibits resistance to pathogens during storage. excellent anti-fungal and anti-inflammatory Columbianetin exhibits excellent anti-fungal and activity. anti-inflammatory activity. Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 5 mg, 10 mg, 20 mg Size: 5 mg, 10 mg, 20 mg (+)-Guaiacin (+)-Peusedanol Cat. No.: HY-N2247A Cat. No.: HY-N6063 (+)-Guaiacin is a compound extracted of the bark (+)-Peusedanol is a coumarin isolated from of Machilus wangchiana Chun. (Lauraceae). Peucedanumjaponicum. (+)-Guaiacin shows potent in vitro activities against the release of β-glucuronidase in rat polymorphonuclear leukocytes (PMNs) induced by platelet-activating factor (PAF) . -

Council Decision (Cfsp) 2020/1368
1.10.2020 EN Offi cial Jour nal of the European Union L 318/5 DECISIONS COUNCIL DECISION (CFSP) 2020/1368 of 1 October 2020 amending Decision 2014/145/CFSP concerning restrictive measures in respect of actions undermining or threatening the territorial integrity, sovereignty and independence of Ukraine THE COUNCIL OF THE EUROPEAN UNION, Having regard to the Treaty on European Union, and in particular Article 29 thereof, Having regard to the proposal of the High Representative of the Union for Foreign Affairs and Security Policy, Whereas: (1) On 17 March 2014, the Council adopted Decision 2014/145/CFSP (1). (2) On 23 December 2019, the President of the Russian Federation, Vladimir Putin, announced the opening of a railway bridge over the Kerch Strait, which marked the implementation of the broader project of connecting the railway infrastructures of the illegally annexed Crimean Peninsula and those of Russia. (3) The Union does not recognise the illegal annexation of Crimea and Sevastopol by the Russian Federation. The construction of the bridge and railway tracks connecting it to the pre‐existing transport infrastructure aims at consolidating the Russian Federation’s control over the illegally annexed Crimea and Sevastopol and further isolating the peninsula from Ukraine. (4) The Council considers that four entities and two individuals should be added to the list of persons, entities and bodies subject to restrictive measures as set out in the Annex to Decision 2014/145/CFSP for their role in designing, building or using the railway infrastructure linking Russia and the illegally annexed Crimean peninsula. (5) The Annex to Decision 2014/145/CFSP should therefore be amended accordingly, HAS ADOPTED THIS DECISION: Article 1 The persons and entities listed in the Annex to this Decision shall be added to the list set out in the Annex to Decision 2014/145/CFSP. -

Yakutia) December 13/2016 Acad
1 61 8 ЯКУТСКИЙ МЕДИЦИНСКИЙ ЖУРНАЛ YAKUT MEDICAL SCIENTIFIC - PRACTICAL JOURNAL OF THE YAKUT SCIENCE CENTRE JOURNAL OF COMPLEX MEDICAL PROBLEMS ISSN 1813-1905 (print) ISSN 2312-1017 (online) 1(61) `2018 ЯКУТСКИЙ МЕДИЦИНСКИЙ ЖУРНАЛ The founder The Yakut Science Centre of Complex Medical Problems YAKUT Editor- in- chief Romanova A.N., MD Editorial Board: MEDICAL Deputy Chief Editor and Executive secretary Nikolaev V.P., MD Scientifc editor JOURNAL Platonov F.A. MD Editorial Council: SCIENTIFIC - PRACTICAL JOURNAL Aftanas L.I., MD, Professor, OF THE YAKUT SCIENCE CENTRE OF COMPLEX acad. RAMS (Novosibirsk) MEDICAL PROBLEMS Voevoda M.I., MD, Professor, Corresponding Member RAMS (Novosibirsk) Ivanov P.M., MD, Professor (Yakutsk) Kryubezi Eric, MD, Professor (France) Quarterly Maksimova N.R., MD (Yakutsk) Mironova G.E., Doctor of Biology, Registered by the Offce of the Federal Service on Professor (Yakutsk) supervision in the feld of communications, information Mikhailova E.I., Doctor of Pedagogics, Professor (Yakutsk) technologies and mass communications in the Republic Nikitin Yu.P., MD, Professor, Sakha (Yakutia) December 13/2016 Acad. RAMS (Novosibirsk) Odland John, MD, Professor (Norway) Registration number PI No.ТU 14-00475 Puzyrev V.P., MD, Professor, Acad. RAMS (Tomsk) Subscription index: 78781 Reutio Arya, MD, PhD, Professor (Finland) Fedorova S.A., Doctor of Biology (Yakutsk) Free price Husebek Anne, MD, Professor (Norway) Khusnutdinova E.K., Doctor of Biology, Professor (Ufa) «Yakut Medical Journal» is included in the approved by Editors: the Higher Attestation Commission of the Russian Chuvashova I.I., Federation List of leading peer-reviewed scientifc Kononova S.l. journals and publications, in which the main scientifc Semenova T.F.