History and Current Status of Plasmodium Falciparum Antimalarial Drug Resistance in Madagascar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Bank Document
1 PID THE WORLwDBANX GROUP ANVorld Free of Poyorty ?lhfoShop Public Disclosure Authorized 'Me Woddl Iank Report No AB84 Initial Project Information Document (PID) Project Name MADAGASCAR-MG-TRANSPORT INFRASTRUCTURE fNVESTMENT PROJECT Region Africa Regional Office Sector Roads and highways (82%); Ports; waterways and shipping(l4%); Aviation (4%) Project ID P082806 Supplemental Project Public Disclosure Authorized Borrower(s) REPUBLIC OF MADAGASCAR Implementing Agency MINISTRY OF TRANSPORT AND MINISTRY OF PUBLIC WORKS Address Program Executive Secretariat Address' Vice Premier Office of Economic Programs, Ministry of Transport, Public Works and Regional Planning, Antananarivo, Madagascar Contact Person Jean Berchmans Rakotomaniraka Tel. 261 33 11 159 42 Fax. Email rjb_sepst@dts mg Environment Category A Date PID Prepared May 15, 2003 Auth Appr/Negs Date September 10, 2003 Bank Approval Date November 13, 2003 1. Country and Sector Background Public Disclosure Authorized The transport sector plays a key role in Madagascar's growth and poverty alleviation strategy. Increased foreign investment, development of the country's eco-tourism and mining potential, and growth in agricultural output all depend on the efficiency of transport services and the availability of appropriate transport infrastructure. Unfortunately, three decades (1970-2000) of inappropriate sector policies have led to a serious deterioration of the country's transport infrastructure. It is estimated that during that period the country lost on average about 1000 kilometers -

PLAGUE OUTBREAK Madagascar External Situation Report 06
PLAGUE OUTBREAK Madagascar External Situation Report 06 Date of issue: 26 October 2017 ....................... ....................... ....................... Grade Cases Deaths CFR 1. Situation update 2 1 309* 93* 7% WHO continues to support the Ministry of Public Health and other national authorities in Madagascar to monitor and respond to the outbreak of plague. The number of new cases of pulmonary plague has continued to decline in all active areas across the country. In the past two weeks, 12 previously affected districts reported no new confirmed or probable cases of pulmonary plague. From 1 August to 24 October 2017, a total of 1 309 suspected cases of plague, including 93 deaths (7%), were reported. Of these, 882 (67%) were clinically classified as pulmonary plague, 221 (17%) were bubonic plague, 1 was septicaemic, and 186 were unspecified (further classification of cases is in process). Since the beginning of the outbreak, 71 healthcare workers (with no deaths) have been affected. Of the 882 clinical cases of pneumonic plague, 235 (27%) were confirmed, 300 (34%) were probable and 347 (39%) remain suspected (additional laboratory results are in process). Fourteen strains of Yersinia pestis have been isolated and were sensitive to antibiotics recommended by the National Program for the Control of Plague. Between 1 August and 24 October 2017, 29 districts have reported confirmed and probable cases of pulmonary plague. The number of districts that reported confirmed and probable cases of pulmonary plague during the last two weeks reduced to 17. About 70% (3 467) of 4 990 contacts identified have completed their 7-day follow-up and a course of prophylactic antibiotics. -

Lessons Learnt from the 2017/2018 Plague Outbreak in Madagascar
Lessons learnt from the 2017/2018 plague outbreak in Madagascar Pr André SPIEGEL and Plague IPM Group Directeur de l’Institut Pasteur de Madagascar 29th European Society of Clinical Microbiology and Infectious Diseases Symposium Global health: African update ESCMIDAmsterdam, Netherlands eLibrary 3th-16th April 2019 © by author Plague Y pestis / rodents / fleas and… humans Rodents Humans and Secondary fleas Pneumonic form Yersinia Person to person transmission pestis Possible evolution to Flea Bubonic form Infected rodent ESCMID eLibrary 2 © by author Plague in Madagascar 1898 : introduction of the Plague in Madagascar (Toamasina) • 1898 : Toamasina epidemic • 24 november 1898 : 1th cas • 1921 : Antananarivo • Following the construction railways • Spread in Madagascar • Endemic to the present day ESCMID eLibrary 3 © by author Plague in Madagascar Rural / seasonal / bubonic • Endemic area • Rural areas of the Central highlands • Natural foci of plague • Altitude > 800 m ESCMID eLibrary 4 © by author Plague in Madagascar Rural / seasonal / bubonic : Reservoirs and Vectors (1) (1) (2) Urban coast Rural area C C (1) B B A A B B (2) Urban highland ESCMID(1) (2) eLibrary(1) A : Rattus rattus (1) : Xenopsylla cheopis B : Rattus norvegicus (2) : Synopsyllus fonquerniei 5 C : Suncus©murinus by author Plague in Madagascar Rural / seasonal / bubonic • Seasonal : October to April • Cases • 500 / year • Bubonic form predominant > 85% notified cases (1998 – 2016) 1998 - 2016 ESCMID eLibrary 6 (Andrianaivoarimanana© V et al,by EID 2019,25,220 -228)author -

The Case of Madagascar
water Article Environmental Factors and the Microbial Quality of Urban Drinking Water in a Low-Income Country: The Case of Madagascar Alexandra Bastaraud 1, Jean Marius Rakotondramanga 2, Jackson Mahazosaotra 1, Noror Ravaonindrina 1 and Ronan Jambou 3,* 1 Food Hygiene and Environment Laboratory, Institut Pasteur of Madagascar, 101 Antananarivo, Madagascar; [email protected] (A.B.); [email protected] (J.M.); [email protected] (N.R.) 2 Epidemiology Department Institut Pasteur of Madagascar, 101 Antananarivo, Madagascar; [email protected] 3 Parasites and Vectors Insects Department, Institut Pasteur, 75015 Paris, France * Correspondence: [email protected]; Tel.: +225-77-92-32-04 Received: 25 July 2018; Accepted: 9 October 2018; Published: 15 October 2018 Abstract: Access to piped water is often limited to urban areas in low-income countries, and the microbiological quality of drinking water varies due to technical and environmental constraints. To analyse the parameters that modulate the contamination of these systems, this study examines 16 years of microbial quality data for water supplied in 32 urban areas of Madagascar. A discriminant statistical approach and agglomerative hierarchical clusters were applied to environmental and climatic data. The microbial contamination varied between sites from 3.3 to 17.5%, and 78% of the supply systems showed large variations between years or months. Agglomerative hierarchical clusters (AHCs) revealed four supply system profiles that share a similar bacteriological evolution. Heavy rainfall and dry periods sustained increasing contamination, as reflected in levels of spores of sulphite-reducing clostridia (SSRC) and/or total coliforms (TC). SSRC were dominant in three profiles, with faecal indicator bacteria (FIB) dominant in the other. -

11Th Indian Ocean Ports and Shipping 2017
Conférence Bilingue Avec Follow us on: Traduction Anglais-Français French to English Bilingual Conference Translation Hotel Carlton, Antananarivo, Madagascar Associate Members Wednesday 22 and Thursday 23 March 2017 Hosted By Organised By Sponsored By • Technical Site Visit • 30 International Exhibition Stands • 35 International Conference Speakers • 300 Conference Delegates • Networking Welcome Reception • Networking Welcome Dinner • Special Offer: Conference Delegate Registration for Shipping Lines; Port Authorities And Terminal Operating Companies at only €795! Save €500! • FREE Conference Delegate Registration for Shippers/Beneficial Cargo Owners (BCOs) KEY SPEAKERS.... PLUS MANY MORE! HIGHLIGHTED TOPICS 1. Christian Eddy Avellin • Indian Ocean economic transformations and trade outlook for 2017: Opportunities and challenges Director General, Port of Toamasina (SPAT), Madagascar from a regional and global perspective 2. Rachid Baho Chief Executive Officer, Madagascar International Container Terminal Services Limited • The Port of Toamasina and its future prospects (MICTSL), Madagascar • Creating value for Eastern and Southern Africa ports: Roles of hinterland countries 3. Philippe Murcia Strategic approach in increasing container handling capacity in Eastern Africa Founder, Ocean Company Consulting, Advisor, ENAC Group, Expert in Maritime Economics, • UNECA (United Nations Economic Commission for Africa), Madagascar • Port investment and expansion opportunities around the Indian Ocean region: Current 4. Nishal Sooredoo developments -

Manandona Power Plant Study.Pdf
Antananarivo Antsirabe Manandona Hydropower Plant Location Map List of Contents Executive Summary .....................................................................................................ES-1 Chapter 1 Introduction................................................................................................1-1 1.1 Background and Objectives...................................................................................... 1-1 1.1.1 Background of the Study........................................................................................... 1-1 1.1.2 Objectives of the Study............................................................................................. 1-6 1.2 Scope of Works......................................................................................................... 1-7 1.3 Study Area................................................................................................................ 1-7 1.4 Study Schedule ......................................................................................................... 1-7 1.5 Study Team Member................................................................................................. 1-7 Chapter 2 Socio-economic Condition in Madagascar ...........................................2-1 2.1 People and Culture.................................................................................................... 2-1 2.2 Economy................................................................................................................... 2-1 -

Port Development in Africa
(C) AfricanBank 2010 Ch2 8/10/10 10:31 Page 31 CHAPTER 2 Port Development in Africa With approximately 80 percent of world how ports perform is an essential element of merchandise trade carried by ships, maritime overall trade costs, as identified in Chapter 1. transport remains by far the most common This is especially the case for Africa, as 15 of mode of international freight transport. It is its countries are landlocked and face severe the backbone to facilitating international infrastructural and trade facilitation trade, offering the most economical and problems. For the landlocked nations, ports reliable way to move goods over long — together with the inland waterway and distances. Ships can carry large volumes of land infrastructures (railroads and highways) merchandise and use free highways in the — constitute a crucial link to the outside seas, which only require infrastructure world and to the global marketplace. investments at the seaports. For all countries, Consequently, high transport-related costs (C) AfricanBank 2010 Ch2 8/10/10 10:31 Page 32 32 African Development Report 2010 represent a fundamental constraint to these This chapter assesses port development LLDCs’ global competitiveness and their and performance throughout Africa (Annex sustained economic growth. 2.1 gives a detailed description of seaports It is generally recognized that the African across the continent). It establishes the areas continent lacks natural ports, while its where improvements in port logistics and, artificial seaports have been poorly more generally, infrastructure, are urgently developed (UNCTAD, 1999; Wood, 2004; needed. However, port development in its Hoyle, 1999). African ports became more broadest sense covers not only the develop- congested following the rise in GDP growth ment of infrastructure and superstructure, and levels of global trade witnessed in most but also environmental concerns. -

List of Medical Facilities/Practitioners in Madagascar
List of Medical Facilities/Practitioners in Madagascar Prepared by British Embassy Antananarivo www.gov.uk The following list of medical facilities/practitioners has been prepared by the British Embassy Antananarivo for the convenience of British Nationals who may require these services and assistance in Madagascar. It is provided on the understanding that we (the British Embassy) do not assume or undertake any legal responsibility, to you, or those affected, if you choose to take it into account when instructing a medical facility or practitioner. Further and alternatively, we cannot accept any liability to any person or company for any financial loss or damage arising from the use of this information or from any failure to give information. Our aim is to provide our customers with as much relevant information to enable them to make better informed decisions but our lists are not recommendations and should not be treated as such. List of medical facilities/practitioners in Madagascar Updated: December 2016 ANTANANARIVO Medical facilities Clinique et Maternité St.François d’Assise (Clinique des Sœurs) Lot VI 83 Ter Mandialaza Ankadifotsy Antananarivo 101 Telephone:+261 (0) 20 22 235 54/ +261 (0) 20 22 695 20/ +261 (0) 33 11 507 25 / +261 (0) 34 41 487 34 This company has told us the following things: They have some English speaking staff It’s a private facility They are affiliated to the Ministry of Health Specialisation in childbirth, minor surgery, general medicine They have experience of representing British nationals You will need to pay for treatment Staff speak Malagasy, French, some English Clinique Médicale Adventiste Soamanandrariny - Antananarivo 101 Dr. -

11973047 01.Pdf
PREFACE In response to a request from the Government of the Republic of Madagascar, the Government of Japan decided to conduct the Feasibility Study on Toamasina Port Development and entrusted the study to the Japan International Cooperation Agency(JICA). JICA selected and dispatched a study team headed by Mr. Kunita of OCDI consists of OCDI, ECOH CORPORATION, and Ides Inc., between January,2009 and December,2009. The team conducted field surveys at the study area, numerical simulation, and held discussions with the responsible persons concerned of the Government of Madagascar and SPAT. Upon returning to Japan,the team prepared this final report. I hope that this report will contribute to the promotion of this project and to the enhancement of prosperous relationship between two countries. Finally,I wish to express my sincere appreciation to the officials concerned of the Government of Madagascar for their close cooperation extended to the study. December 2009 Toshiyuki Kuroyanagi Director General Economic Infrastructure Department Japan international Cooperation Agency Toamasina Port ( Urgent Development Plan ) LIST OF ABBREVIATIONS A ANGAP National Association for Management of Protected Areas APMF Agence Portuaire Maritime et Fluviale B BOT Build Operate Transfer BP Balance of Payment C CF Conversion Factor CIF Cost, Insurance and Freight CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora CO2 Carbon dioxide CTE Technical Evaluation Committee CY Container Yard D DEE Department of Environmental Evaluation -
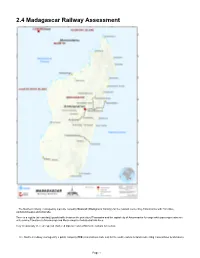
2.4 Madagascar Railway Assessment
2.4 Madagascar Railway Assessment - The Northern railway, managed by a private company Madarail (Madagascar Railway) for the network connecting Antananarivo with Tamatave, Ambatondrazaka and Antsirabe. There is a regular (at least daily) goods traffic between the port city of Toamasina and the capital city of Antananarivo for cargo while passenger trains are only serving Tamatave to Moramanga and Moramanga to Ambatrodrazaka lines. Very occasionally there are special chartered trips on restored Micheline railcars for tourists. - The Southern railway, managed by a public company FCE (Fianarantsoa Cote Est) for the south eastern network connecting Fianarantsoa to Manakara. Page 1 The southern line has regular passenger and cargo trains, which provides a slow but picturesque alternative to the recently rehabilitated road in the region. For more information on railway company contact details, please see the following link: Madagascar Railway Assessment Railway Companies and Consortia 4.2.7 Madagascar Railway Company Contact List Northern railway*: *During our study, Madarail was in the midst of restructuring, therefore, they did not want to share information, statistics or even contacts. All the information gathered and shared in this document comes exclusively from third parties or from data found on the internet. Madarail, was founded on October 10, 2002 following the decision of the Malagasy State to privatize the Malagasy National Railway Network1 (RNCFM). A concession agreement for the management of the North network is then established between the new private operator and the State. Madarail began operating the Northern railway network in Madagascar on 1 July 2003. In 2008, the Belgian operator Vecturis, already active in eight other African countries, became the majority shareholder of the company and the new railway operator. -

The Project on Master Plan Formulation for Economic Axis of Tatom (Antananarivo-Toamasina, Madagasikara)
Ministry of Regional Development, Building, Housing and Public Works (MAHTP) Government of the Republic of Madagascar The Project on Master Plan Formulation for Economic Axis of TaToM (Antananarivo-Toamasina, Madagasikara) Final Report Summary October 2019 Japan International Cooperation Agency (JICA) Oriental Consultants Global Co., Ltd. CTI Engineering International Co., Ltd. CTI Engineering Co., Ltd. EI JR 19-102 Ministry of Regional Development, Building, Housing and Public Works (MAHTP) Government of the Republic of Madagascar The Project on Master Plan Formulation for Economic Axis of TaToM (Antananarivo-Toamasina, Madagasikara) Final Report Summary October 2019 Japan International Cooperation Agency (JICA) Oriental Consultants Global Co., Ltd. CTI Engineering International Co., Ltd. CTI Engineering Co., Ltd. Currency Exchange Rates EUR 1.00 = JPY 127.145 EUR 1.00 = MGA 3,989.95 USD 1.00 = JPY 111.126 USD 1.00 = MGA 3,489.153 MGA 1.00 = JPY 0.0319 Average during the period between June 2018 and June 2019 Administrative Divisions of Madagascar The decentralised administrative divisions of Madagascar is divided into 22 regions which are further divided into 114 districts. The districts are further divided into communes and each communes into fokontany. Besides the decentralised administrative divisions, the country is subdivided into six provinces, divided into 24 prefectures. The prefectures are divided into 117 districts and further into arrondissements. The boundary of region and prefecture are same except for two prefectures Nosy -

Faritany Region District Commune
REPOBLIKAN’I MADAGASIKARA Fitiavana – Tanindrazana – Fandrosoana COMMISSION ELECTORALE NATIONALE INDEPENDANTE _______________ ANNEXE DE LA DELIBERATION N°012 /CENI/D/2020 DU 12 OCTOBRE 2020 Fixant la liste et l’emplacement des bureaux de vote pour les élections sénatoriales de 2020 FARITANY REGION DISTRICT COMMUNE FOKONTANY CV CODE BV EMPLACEMENT BV ANTANANARIVO ANALAMANGA AMBOHIDRATRIMO AMBOHIDRATRIMO AMBOHIDRATRIMO TRANOMPOKONOLONA 110101 TRANOMPOKONOLONA ANTANANARIVO ANALAMANGA ANDRAMASINA ANDRAMASINA ANDRAMASINA TRANOMPOKONOLONA ANDRAMASINA 110201 TRANOMPOKONOLONA ANTANANARIVO ANALAMANGA ANJOZOROBE ANJOZOROBE ANJOZOROBE TRANOMPOKONOLONA 110301 TRANOMPOKONOLONA ANTANANARIVO ANALAMANGA ANKAZOBE ANKAZOBE ANKAZOBE I BUREAU FKT ANKAZOBE I 110401 BUREAU FKT ANKAZOBE I ANTANANARIVO ANALAMANGA ANTANANARIVO-ATSIMONDRANO BEMASOANDRO ANOSIMASINA COMMUNE 110501 SALLE DE MARIAGE ANTANANARIVO ANALAMANGA ANTANANARIVO AVARADRANO SABOTSY NAMEHANA NAMEHANA LAPAN'NY TANANA 110601 LAPAN'NY TANANA ANTANANARIVO ANALAMANGA ANTANANARIVO-RENIVOHITRA C.U ANTANANARIVO NANISANA BUREAU CER ANALAMANGA 110701 BUREAU CER ANALAMANGA ANTANANARIVO ANALAMANGA MANJAKANDRIANA C.U MANJAKANDRIANA MANJAKANDRIANA BUREAU CED 111301 BUREAU CED ANTANANARIVO BONGOLAVA FENOARIVOBE FENOARIVOBE FENOARIVOBE TRANOMPOKONOLONA 120101 TRANOMPOKONOLONA ANTANANARIVO BONGOLAVA TSIROANOMANDIDY C.U TSIROANOMANDIDY TSIROANOMANDIDY ATSIMO BUREAU CED 120201 BUREAU CED ANTANANARIVO ITASY ARIVONIMAMO Arivonimamo I Arivonimamo Centre Lapan'ny Tanana Arivonimamo I 130101 Salle de mariage ANTANANARIVO