Acral Manifestations of Soft Tissue Tumors Kristen M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Best Diagnosis Is
Dermatopathology Diagnosis Superficial Plantar Fibromatosis The best diagnosis is: H&E, original magnification 40. a. dermatofibroma b. keloid CUTISc. neurofibroma d. nodular fasciitis Do Note. superficialCopy plantar fibromatosis H&E, original magnification 400. PLEASE TURN TO PAGE 225 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Luke Lennox, BA; Anna Li, BS; Thomas N. Helm, MD Mr. Lennox and Dr. Helm are from the Department of Dermatology, University at Buffalo, The State University of New York. Ms. Li is from the Department of Dermatology, Ross University, Dominica, West Indies. The authors report no conflict of interest. Correspondence: Thomas N. Helm, MD, Dermatopathology Laboratory, 6255 Sheridan Dr, Building B, Ste 208, Williamsville, NY 14221 ([email protected]). 220 CUTIS® WWW.CUTIS.COM Copyright Cutis 2013. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Superficial Plantar Fibromatosis lantar fibromatosis typically presents as firm represents a reactive proliferation of spindle cells most plaques or nodules on the plantar surface of the often encountered on the extremities of young adults. Pfoot.1 The process is caused by a proliferation of Spindle cells are loosely arranged in a mucinous fibroblasts and collagen and has been associated with stroma and are not circumscribed (tissue culture ap- trauma, liver disease, diabetes mellitus, epilepsy, and pearance). Vesicular nuclei are encountered, but there alcoholism.2 Unlike the fibromatoses associated with is no remarkable nuclear pleomorphism. Extravasated Gardner syndrome, superficial plantar fibromatosis has not been associated with abnormalities in the ade- nomatous polyposis coli gene or with the -catenin gene.3,4 Lesions typically present in middle-aged or elderly individuals and involve the medial plantar fas- cia. -

A Follow-Up of Patient Reported Outcomes in Chronic Plantar Heel Pain Participants Treated with Graston Technique: a Mixed Methods Approach
University of Northern Iowa UNI ScholarWorks Dissertations and Theses @ UNI Student Work 2016 A follow-up of patient reported outcomes in chronic plantar heel pain participants treated with Graston Technique: A mixed methods approach Troy Richard Garrett University of Northern Iowa Let us know how access to this document benefits ouy Copyright ©2016 Troy Richard Garrett Follow this and additional works at: https://scholarworks.uni.edu/etd Part of the Physical Therapy Commons, and the Physiotherapy Commons Recommended Citation Garrett, Troy Richard, "A follow-up of patient reported outcomes in chronic plantar heel pain participants treated with Graston Technique: A mixed methods approach" (2016). Dissertations and Theses @ UNI. 344. https://scholarworks.uni.edu/etd/344 This Open Access Dissertation is brought to you for free and open access by the Student Work at UNI ScholarWorks. It has been accepted for inclusion in Dissertations and Theses @ UNI by an authorized administrator of UNI ScholarWorks. For more information, please contact [email protected]. Copyright by TROY RICHARD GARRETT 2016 All Rights Reserved A FOLLOW-UP OF PATIENT REPORTED OUTCOMES IN CHRONIC PLANTAR HEEL PAIN PARTICIPANTS TREATED WITH GRASTON TECHNIQUE: A MIXED METHODS APPROACH An Abstract of a Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree Doctor of Education Approved: ____________________________________ Dr. Peter J. Neibert, Committee Chair ____________________________________ Dr. Kavita R. Dhanwada Dean of the Graduate College Troy Richard Garrett University of Northern Iowa December 2016 ABSTRACT Chronic Plantar Heel Pain (CPHP), commonly known as “plantar fasciitis,” is a condition that is estimated to affect 10% of the American population. -

Pathology and Genetics of Tumours of Soft Tissue and Bone
bb5_1.qxd 13.9.2006 14:05 Page 3 World Health Organization Classification of Tumours WHO OMS International Agency for Research on Cancer (IARC) Pathology and Genetics of Tumours of Soft Tissue and Bone Edited by Christopher D.M. Fletcher K. Krishnan Unni Fredrik Mertens IARCPress Lyon, 2002 bb5_1.qxd 13.9.2006 14:05 Page 4 World Health Organization Classification of Tumours Series Editors Paul Kleihues, M.D. Leslie H. Sobin, M.D. Pathology and Genetics of Tumours of Soft Tissue and Bone Editors Christopher D.M. Fletcher, M.D. K. Krishnan Unni, M.D. Fredrik Mertens, M.D. Coordinating Editor Wojciech Biernat, M.D. Layout Lauren A. Hunter Illustrations Lauren A. Hunter Georges Mollon Printed by LIPS 69009 Lyon, France Publisher IARCPress International Agency for Research on Cancer (IARC) 69008 Lyon, France bb5_1.qxd 13.9.2006 14:05 Page 5 This volume was produced in collaboration with the International Academy of Pathology (IAP) The WHO Classification of Tumours of Soft Tissue and Bone presented in this book reflects the views of a Working Group that convened for an Editorial and Consensus Conference in Lyon, France, April 24-28, 2002. Members of the Working Group are indicated in the List of Contributors on page 369. bb5_1.qxd 22.9.2006 9:03 Page 6 Published by IARC Press, International Agency for Research on Cancer, 150 cours Albert Thomas, F-69008 Lyon, France © International Agency for Research on Cancer, 2002, reprinted 2006 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. -

Mast Cell Infiltration and Leukotriene
Journal of Cancer Therapy, 2015, 6, 606-612 Published Online July 2015 in SciRes. http://www.scirp.org/journal/jct http://dx.doi.org/10.4236/jct.2015.67066 Mast Cell Infiltration and Leukotriene Receptor Expression in Various Tumors: Possible Clinical Application of Common Pathological Findings Concerned with Tumor Development Masao Sugamata*, Tomomi Ihara, Miho Sugamata Department of Pathology, Tochigi Institute of Clinical Pathology, Tochigi, Japan Email: *[email protected] Received 18 June 2015; accepted 18 July 2015; published 21 July 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Since the mechanisms of the developmental processes of tumors remain unclear, early detection and early treatment of the tumors is necessary to save patients with malignant tumors. Therapies currently available to patients are surgery, chemotherapy, and radiotherapy. However, there are many patients who cannot be saved by such therapies. In this study, we found the common fea- tures of various tumor tissues, and we demonstrated the effect of therapeutics that target them by using experimental rats with spontaneous tumors. 26 kinds of human tumors (epithelial or me- senchymal origin, and malignant or benign) and Sprague-Dawley rats with spontaneous mammary gland tumors were examined by light and electron microscope. To detect of mast cells and leuko- triene receptor, toluidine blue stain and immunohistochemical stain were performed. The rats were orally administered one of leukotriene receptor antagonists. We found that the presence of numerous mast cells and expression of leukotriene receptors in various tumor (human tumors and rat spontaneous tumors). -

A Pilot Study of a Novel Treatment Method for Refractory Painful Plantar Fibromas
Open Access Austin Journal of Orthopedics & Rheumatology Research Article A Pilot Study of a Novel Treatment Method for Refractory Painful Plantar Fibromas Mihir M. Patel*, Sahvan M. Patel, Sia S. Patel and Jacob Daynes Abstract Department of Orthopedic Surgery, OrthoIndy, USA Introduction: Painful plantar fibromas may make ambulation difficult *Corresponding author: Mihir M Patel, Department for patients. They may recur and can make shoe wear difficult to purchase. of Orthopedic Surgery, OrthoIndy 8450 Northwest Blvd, Treatment modalities may include conservative care, modified shoe wear, Indianapolis, Indiana 46278, USA injections and orthotics. Surgical treatment may include open excision. The TX1A probe by Tenex Inc may be a useful alternative treatment modality for Received: March 18, 2015; Accepted: April 22, 2015; these painful lesions. Published: April 22, 2015 Materials and Methods: From 2011 through 2014, eight patients who had painful plantar fibromas elected to undergo definitive treatment for the fibromas. They all had had conservative care including modifications of shoes and either over the counter orthotics or custom orthotics. They all had advanced imaging tests (MRI or CT) preoperatively to help delineate the lesions. The definitive treatment utilized arthroscopy and the TX1A probe and was performed on an outpatient basis. Results: Preoperative AOFAS score was 30.8 (20-36). Postoperative AOFAS score was 90.1 (85-92). Average time of resolution of symptoms was 63.5 days (30-112) and average follow-up was 2.5 years. One patient had another lesion become painful but it was in a different location than the index operation. No others have had a recurrence to date. -

Integrated Data Analysis Reveals Uterine Leiomyoma Subtypes with Distinct Driver Pathways and Biomarkers
Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers Miika Mehinea,b, Eevi Kaasinena,b, Hanna-Riikka Heinonena,b, Netta Mäkinena,b, Kati Kämpjärvia,b, Nanna Sarvilinnab,c, Mervi Aavikkoa,b, Anna Vähärautiob, Annukka Pasanend, Ralf Bützowd, Oskari Heikinheimoc, Jari Sjöbergc, Esa Pitkänena,b, Pia Vahteristoa,b, and Lauri A. Aaltonena,b,e,1 aMedicum, Department of Medical and Clinical Genetics, University of Helsinki, Helsinki FIN-00014, Finland; bResearch Programs Unit, Genome-Scale Biology, University of Helsinki, Helsinki FIN-00014, Finland; cDepartment of Obstetrics and Gynecology, Helsinki University Hospital, University of Helsinki, Helsinki FIN-00029, Finland; dDepartment of Pathology and HUSLAB, Helsinki University Hospital, University of Helsinki, Helsinki FIN-00014, Finland; and eDepartment of Biosciences and Nutrition, Karolinska Institutet, SE-171 77, Stockholm, Sweden Edited by Bert Vogelstein, Johns Hopkins University, Baltimore, MD, and approved December 18, 2015 (received for review September 25, 2015) Uterine leiomyomas are common benign smooth muscle tumors that with deletions affecting collagen, type IV, alpha 5 and collagen, type impose a major burden on women’s health. Recent sequencing studies IV, alpha 6 (COL4A5-COL4A6) may constitute a rare fourth subtype have revealed recurrent and mutually exclusive mutations in leiomyo- (4). HMGA2 and MED12 represent the two most common driver mas, suggesting the involvement of molecularly distinct pathways. In genes and together contribute to 80–90% of all leiomyomas (5). this study, we explored transcriptional differences among leiomyomas Less frequently, leiomyomas harbor 6p21 rearrangements af- harboring different genetic drivers, including high mobility group fecting high mobility group AT-hook 1 (HMGA1) (6). -
346 Fetal Cardiac Rhabdomyoma: a Case Report
A CASE REPORT Bali Medical Journal (Bali Med J) 2016, Volume 5, Number 3: 543-546 P-ISSN.2089-1180, E-ISSN.2302-2914 Fetal cardiac rhabdomyoma: a case report A Case Report Tjokorda Gde Agung Suwardewa,1* I Ketut Surya Negara,1 AAN Jaya Kusuma,1 AAP Wiradnyana,1 Ryan Mulyana Surya,1 I Ketut Tunas2 CrossMark Doi: http://dx.doi.org/10.15562/bmj.v5i3.346 Published by DiscoverSys ABSTRACT Volume No.: 5 Background: Fetal cardiac rhabdomyoma is a rare condition. tomography scan, to rule out anything related to tuberous sclerosis. Case: We report a case with cardiac mass discovered in utero by The prognosis depends on the size, site, number of tumors, and co- prenatal ultrasonography at 33 weeks of gestational age. An echogenic existing congenital abnormalities. Management highly depends on Issue: 3 round-oval shape mass at the interventricular septum protrudes to left the presence of outflow tract obstruction of the heart. However, some ventricle was observed. cases may regress after birth. Results: After birth, the baby was followed up for 7 months with echocardiography, physical examination, and computerized First page No.: 543 Keywords: rhabdomyoma, fetal heart, tuberous sclerosis. Cite This Article: Suwardewa, T., Surya Negara, I., Jaya Kusuma, A., Wiradnyana, A., Surya, R., Tunas, I. 2016. Fetal cardiac rhabdomyoma: a case P-ISSN.2089-1180 report. Bali Medical Journal 5(3): 543-546. DOI:10.15562/bmj.v5i3.346 1Department of Obstetrics and INTRODUCTION rhabdomyoma, which found incidentally during a E-ISSN.2302-2914 Gynecology, Udayana University / routine antenatal visit. Sanglah Hospital Denpasar, Bali- The presence of a primary congenital tumor of Indonesia. -

Plantar Fasciitis Standard of Care
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Plantar Fasciitis Case Type / Diagnosis: (diagnosis specific, impairment/ dysfunction specific) ICD9 - 728.71 plantar fibromatosis Plantar fasciitis is an inflammatory condition that occurs as a result of overstressing the plantar fascia. It is the most common cause of inferior heel pain and has been diagnosed in patients from the ages of 8-80. 1-4 Plantar fasciitis affects approximately 10% of the population and is more commonly found in middle-aged women and younger male runners.2, 3, 5 A majority of the patients diagnosed with this inflammatory condition are over the age of 40.3 Bilateral symptoms can occur in 20-30% of those diagnosed with plantar fasciitis.6 However in these cases it is important to rule out other systemic processes such as rheumatoid arthritis, systemic lupus erythematosus, Reiter’s disease, gout and ankylosing spondylitis.3, 6 The primary symptom of plantar fasciitis is pain in the heel when the patient first rises in the morning and when the plantar fascia is palpated over it’s origin at the medial calcaneal tuberosity.2-4, 6, 7 The plantar fascia (aponeurosis) is a thick fibrous band of connective tissue that originates at the medial and lateral tuberosities of the calcaneus. It runs longitudinally and has 3 portions, medial, central, and lateral. At the midfoot level, the fascia divides into 5 separate bands, which blend with the flexor tendon sheaths and transverse metatarsal ligaments and attach at the base of each proximal phalange. The central portion of the plantar fascia is the largest section. -

Two Genetic Variants Associated with Plantar Fascial Disorders
Genetics & Molecular Biology Thieme Two Genetic Variants Associated with Plantar Fascial Disorders Authors Stuart K. Kim1, John P.A. Ioannidis2, Marwa A. Ahmed3, Andrew L. Avins4, John P. Kleimeyer5, Michael Fredericson5, Jason L. Dragoo5 Affiliations Stanford, California 94305 1 Dept. Developmental Biology, Stanford University United States Medical Center, Stanford CA, USA Tel.: + 1/650/867 3189, Fax: + 1/650/725 7739 2 Dept. of Medicine, Stanford Prevention Research Center [email protected] and Dept. of Health Research and Policy, Division of Epidemiology, Stanford University School of Medicine, ABSTRACT and Dept. of Statistics, Stanford University School of Plantar fascial disorder is comprised of plantar fasciitis and Humanities and Sciences, Stanford CA, USA plantar fibromatosis. Plantar fasciitis is the most common 3 Dept. Physical Medicine and Rehabilitation, Harvard cause of heel pain, especially for athletes involved in running Medical School, Boston MA, USA and jumping sports. Plantar fibromatosis is a rare fibrous -hy 4 Kaiser Permanente Northern California, Division of perproliferation of the deep connective tissue of the foot. To Research, Oakland, California, USA identify genetic loci associated with plantar fascial disorders, 5 Dept. Orthopaedic Surgery, Stanford University Medical a genome-wide association screen was performed using publi- Center, Stanford CA, USA cally available data from the Research Program in Genes, Envi- ronment and Health including 21,624 cases of plantar fascial Key words disorders and 80,879 controls. One indel (chr5:118704153:D) Genome-wide association study, plantar fasciitus, sports and one SNP (rs62051384) showed an association with plantar injury, plantar fibromatosis fascial disorders at genome-wide significance (p < 5 × 10 − 8) with small effects (odds ratios = 0.93 and 1.07 per allele, re- Bibliography spectively). -

Lipomata of the Uterus
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 1940 Lipomata of the uterus. DeLou Perrin Hall University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Part of the Medicine and Health Sciences Commons Recommended Citation Hall, DeLou Perrin, "Lipomata of the uterus." (1940). Electronic Theses and Dissertations. Paper 2035. https://doi.org/10.18297/etd/2035 This Master's Thesis is brought to you for free and open access by ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. UNIVERSrTY .OF LOUISVILLE LIPOMATA OF THE UTERUS " A Dis.sertation Submitted to the Faculty Of the Graduate School of the University of Louisville In Partial Fulfillment of the Requirements for the Degree Of Master of Science Department .of Pathology By DeLou Perrin Hall,.. Year 1940 '. PREFAOE In the writing of this thesis I have spent many happy hours in search of literature relative to Lipomata of the uterus, the task has been enjoyable because of the ever stimulating influence of the Frofessor of Pathology in the University of Louisville, Dr. A. James Miller, to whom I am grateful. To my associate and erstwhile Professor of Surgery, Dr. J. Garland Sherrill, whose profound wisdom has been a tlLamp unto my- feet and a light unto my- path," my sincere thanks. -

Dermoscopy As an Adjuvant Tool for Detecting Skin Leiomyomas In
Diluvio et al. BMC Dermatology 2014, 14:7 http://www.biomedcentral.com/1471-5945/14/7 CASE REPORT Open Access Dermoscopy as an adjuvant tool for detecting skin leiomyomas in patient with uterine fibroids and cerebral cavernomas Laura Diluvio1*, Claudia Torti1, Alessandro Terrinoni2, Eleonora Candi2, Raffaella Piancatelli3, Emilio Piccione3, Evelin Jasmine Paternò4, Sergio Chimenti1, Augusto Orlandi5, Elena Campione1† and Luca Bianchi1† Abstract Background: Hereditary syndromes frequently need the cooperation of different specialties to increase diagnostic competence. Multiple cutaneous and uterine leiomyomatosis syndrome is a rare autosomal dominant disorder caused by the mutations of the fumarate hydratase gene, demonstrated in 80 to 100 percent of affected individuals. This can be linked to an increased risk of renal cancer in both sexes. The skin involvement is described to highlight the diagnostic role of the cutaneous counterpart in identifying this rare syndrome. Case presentation: A 37-year-old woman suffering from several uterine fibroids presented multiple, painful, papulo-nodules on her left subscapular side, both forearms and legs. The patient underwent surgery on six lesions: five were leiomyomas, whilst one was a dermatofibroma. Genetic sequencing did not evidence known fumarate hydratase gene mutations. Dermoscopy showed a brown delicate pigmented network and included leiomyomas among the non-melanocytic benign skin tumours featuring a dermatofibroma-like pattern. Abdominal computerized-tomography scan did not reveal renal cancer, but brain magnetic resonance imaging showed one asymptomatic cerebral cavernoma. The patient benefited from the surgical removal of the five larger cutaneous lesions and from gabapentin, which relieved her pain. Conclusions: This observation highlights the usefulness of dermoscopy in the diagnosis of cutaneous leiomyomas disclosing multiple cutaneous and uterine leiomyomatosis syndrome. -
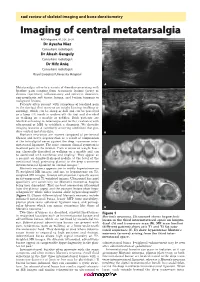
Imaging of Central Metatarsalgia
rad review of skeletal imaging and bone densitometry Imaging of central metatarsalgia RAD Magazine, 45, 528, 29-30 Dr Ayesha Niaz Consultant radiologist Dr Akash Ganguly Consultant radiologist Dr Hifz Aniq Consultant radiologist Royal Liverpool University Hospital A Metatarsalgia refers to a variety of disorders presenting with forefoot pain ranging from traumatic lesions (acute or chronic repetitive), inflammatory and infective disorders, non-neoplastic soft tissue lesions, and benign tumours to malignant lesions. Patients often present with symptoms of localised pain in the forefoot that worsens on weight bearing (walking or running), which can be sharp or dull and can be perceived as a lump felt inside or underneath the foot and described as walking on a marble or pebbles. Such patients are labelled as having metatarsalgia and further evaluated with ultrasound or MRI to establish a diagnosis. We describe imaging features of commonly occurring conditions that pro- duce central metatarsalgia. B Morton’s neuromas are masses composed of perineural fibrosis and nerve degeneration as a result of compression of the interdigital nerve against the deep transverse inter- metatarsal ligament. The most common clinical symptom is localised pain in the forefoot. Pain is worse on weight bear- ing, classically described as walking on a marble and can be associated with numbness and tingling.1 They appear as a peanut- or dumbbell-shaped nodule at the level of the metatarsal head, projecting plantar to the deep transverse intermetatarsal ligament on coronal images.2 Morton’s neuroma appears iso- to mildly hyperintense on C T1-weighted MR images and iso- to hypointense on T2- weighted MR images.