Perspective and Trends in the Development of Science Education and Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 in the COURT of SPECIAL JUDGE, PREVENTION of CORRUPTION ACT, AIZAWL JUDICIAL DISTRICT, AIZAWL. Crl. Trl No. 1511 of 2010 in AC
IN THE COURT OF SPECIAL JUDGE, PREVENTION OF CORRUPTION ACT, AIZAWL JUDICIAL DISTRICT, AIZAWL. Crl. Trl No. 1511 of 2010 in ACB Case No. 2/2007 U/s 409/464/34 IPC R/w Sec. 13(I) (c) (d) and 13 (2) of PC Act, 1988. STATE OF MIZORAM ……………… Complainant VRS 1. Rasik Mohan Chakma 2. Rosendro Singh ……………… Accused persons BEFORE SMT. LUCY LALRINTHARI SPECIAL JUDGE, P.C ACT APPEARANCE For the Prosecution: Mr. H.Lalmuankima, Ld. P.P. For the Accused persons: Mr. S.L.Thansanga, Ld. Advocate Mr. J.N.Bualteng, Ld. Advocate Date of hearing: 3.10.2016 Date of Order: 27.10.2016 JUDGMENT &ORDER 1. The two accused persons above were facing trial in connection with offences punishable under sections U/s 409/464/34 IPC R/w Sec. 13(I) (c) (d) and 13 (2) of PC Act, 1988, and the court deliver the following judgment. Prosecution Story of the Case: 2. The prosecution story of the case in brief is that on 13.11.2007, Inspector Paul Thangzika of ACB (Anti Corruption Branch) lodged a written report to the S.P., ACB that (1) Pulin Bayan Chakma, MDC, CLP (Congress Legislature Party) Leader, ACDC (Autonomous Chakma District Council) and Chairman, Vigilance Committee, Chakma District Congress Committee, Kamalanagar, (2) Sushil Kumar Chakma, Vice President, Chakma District Congress Committee, (3) Nirupam Chakma, Chairman, Minority Department, MPCC, Aizawl and (4) Kalikumar Tongchongya, President, Chakma District Youth Congress Committee, 1 Kamalanagar had submitted a written complaint to His Excellency, the Governor of Mizoram against the authority of Chama Autonomous District Council for dishonestly mis-utilizing the Centrally sponsored Scheme (CSS) under the scheme of Rashtriya Sam Vikash Yojana (RSVY). -

List of Organisations/Individuals Who Sent Representations to the Commission
1. A.J.K.K.S. Polytechnic, Thoomanaick-empalayam, Erode LIST OF ORGANISATIONS/INDIVIDUALS WHO SENT REPRESENTATIONS TO THE COMMISSION A. ORGANISATIONS (Alphabetical Order) L 2. Aazadi Bachao Andolan, Rajkot 3. Abhiyan – Rural Development Society, Samastipur, Bihar 4. Adarsh Chetna Samiti, Patna 5. Adhivakta Parishad, Prayag, Uttar Pradesh 6. Adhivakta Sangh, Aligarh, U.P. 7. Adhunik Manav Jan Chetna Path Darshak, New Delhi 8. Adibasi Mahasabha, Midnapore 9. Adi-Dravidar Peravai, Tamil Nadu 10. Adirampattinam Rural Development Association, Thanjavur 11. Adivasi Gowari Samaj Sangatak Committee Maharashtra, Nagpur 12. Ajay Memorial Charitable Trust, Bhopal 13. Akanksha Jankalyan Parishad, Navi Mumbai 14. Akhand Bharat Sabha (Hind), Lucknow 15. Akhil Bharat Hindu Mahasabha, New Delhi 16. Akhil Bharatiya Adivasi Vikas Parishad, New Delhi 17. Akhil Bharatiya Baba Saheb Dr. Ambedkar Samaj Sudhar Samiti, Basti, Uttar Pradesh 18. Akhil Bharatiya Baba Saheb Dr. Ambedkar Samaj Sudhar Samiti, Mirzapur 19. Akhil Bharatiya Bhil Samaj, Ratlam District, Madhya Pradesh 20. Akhil Bharatiya Bhrastachar Unmulan Avam Samaj Sewak Sangh, Unna, Himachal Pradesh 21. Akhil Bharatiya Dhan Utpadak Kisan Mazdoor Nagrik Bachao Samiti, Godia, Maharashtra 22. Akhil Bharatiya Gwal Sewa Sansthan, Allahabad. 23. Akhil Bharatiya Kayasth Mahasabha, Amroh, U.P. 24. Akhil Bharatiya Ladhi Lohana Sindhi Panchayat, Mandsaur, Madhya Pradesh 25. Akhil Bharatiya Meena Sangh, Jaipur 26. Akhil Bharatiya Pracharya Mahasabha, Baghpat,U.P. 27. Akhil Bharatiya Prajapati (Kumbhkar) Sangh, New Delhi 28. Akhil Bharatiya Rashtrawadi Hindu Manch, Patna 29. Akhil Bharatiya Rashtriya Brahmin Mahasangh, Unnao 30. Akhil Bharatiya Rashtriya Congress Alap Sankyak Prakosht, Lakheri, Rajasthan 31. Akhil Bharatiya Safai Mazdoor Congress, Jhunjhunu, Rajasthan 32. Akhil Bharatiya Safai Mazdoor Congress, Mumbai 33. -

List of Eklavya Model Residential Schools in India (As on 20.11.2020)
List of Eklavya Model Residential Schools in India (as on 20.11.2020) Sl. Year of State District Block/ Taluka Village/ Habitation Name of the School Status No. sanction 1 Andhra Pradesh East Godavari Y. Ramavaram P. Yerragonda EMRS Y Ramavaram 1998-99 Functional 2 Andhra Pradesh SPS Nellore Kodavalur Kodavalur EMRS Kodavalur 2003-04 Functional 3 Andhra Pradesh Prakasam Dornala Dornala EMRS Dornala 2010-11 Functional 4 Andhra Pradesh Visakhapatanam Gudem Kotha Veedhi Gudem Kotha Veedhi EMRS GK Veedhi 2010-11 Functional 5 Andhra Pradesh Chittoor Buchinaidu Kandriga Kanamanambedu EMRS Kandriga 2014-15 Functional 6 Andhra Pradesh East Godavari Maredumilli Maredumilli EMRS Maredumilli 2014-15 Functional 7 Andhra Pradesh SPS Nellore Ozili Ojili EMRS Ozili 2014-15 Functional 8 Andhra Pradesh Srikakulam Meliaputti Meliaputti EMRS Meliaputti 2014-15 Functional 9 Andhra Pradesh Srikakulam Bhamini Bhamini EMRS Bhamini 2014-15 Functional 10 Andhra Pradesh Visakhapatanam Munchingi Puttu Munchingiputtu EMRS Munchigaput 2014-15 Functional 11 Andhra Pradesh Visakhapatanam Dumbriguda Dumbriguda EMRS Dumbriguda 2014-15 Functional 12 Andhra Pradesh Vizianagaram Makkuva Panasabhadra EMRS Anasabhadra 2014-15 Functional 13 Andhra Pradesh Vizianagaram Kurupam Kurupam EMRS Kurupam 2014-15 Functional 14 Andhra Pradesh Vizianagaram Pachipenta Guruvinaidupeta EMRS Kotikapenta 2014-15 Functional 15 Andhra Pradesh West Godavari Buttayagudem Buttayagudem EMRS Buttayagudem 2018-19 Functional 16 Andhra Pradesh East Godavari Chintur Kunduru EMRS Chintoor 2018-19 Functional -

A Baseline Survey of Minority Concentration Districts of India
A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA Lawngtlai (Mizoram) Sponsored by Ministry of Miniority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax : 23765410 Email: [email protected], website:ihdindia.org 2008 A BASELINE SURVEY OF MINORITY CONCENTRATION DISTRICTS OF INDIA Lawngtlai (Mizoram) Sponsored by Ministry of Miniority Affairs Government of India and Indian Council of Social Science Research INSTITUTE FOR HUMAN DEVELOPMENT NIDM Building, 3rd Floor, IIPA Campus I.P Estate, Mahatma Gandhi Marg, New Delhi-110 002 Phones – 2335 8166, 2332 1610 / Fax: 23765410 Email: [email protected], website: www.ihdindia.org RESEARCH TEAM Principal Researchers Alakh N. Sharma Ashok K. Pankaj Data Processing and Tabulation Balwant Singh Mehta Sunil Kumar Mishra Abhay Kumar Research Associates/Field Supervisors Ramashray Singh Ashwani Kumar Subodh Kumar M. Poornima Research Assistant P.K. Mishra Secretarial Assistance Shri Prakash Sharma Nidhi Sharma Sindhu Joshi LAWNGTLAI Principal Authors of the Report Falendra K. Sudan Senior Visiting Fellow, IHD & R.P.Mamgain Senior Fellow, IHD CONTENTS Executive Summary....................................................................................................i-vii Chapter I: Introduction ..............................................................................................1-10 Chapter II: Village Level Deficits.............................................................................11-15 -

The Mizoram Gazette Published by Authority
Regd. No. NE 907 • The Mizoram Gazette Published by Authority VOL. xxv Aizawl Friday, 1. 11. 1996 Kartika 10, S.E. 1918 Issue No. 44 Government of Mizoram Part I Appointments, Postings, Transfers, Powers, Leave and Other Personal Notioes and Order&. • (ORDERS BY THE GOVERNOR) I • ! • NOTIFICATIO NS • No. A. 19015j1� 196-VIG the 1st November, 1996. On the expiry of his re-employ ment as Deputy Superintendent of Police, Anti-Corruption Branch for period of 4 (four) months with effect from 1.7.1996 to 31.10 1996, Pu R. Doliana, Deputy Superintendent of Police, Anti-Corruption Branch is released from the office of Superintendent of Police, Anti-Corruption Branch on 31.10.96(AjN). T. Sangkunga, Deputy Secretary to the Govt. of Mizoram, Vigilance Department. No. A. 33012jlj96-HFW(L) the 28th October, 1996. The Governor of Mizoram is pleased to retire and release Pi Thanpari Pautu, Dy. Director (Nursing), Mizoram Aizawl who bas attained the age of superannuation retirement with effect from 31. 10. 1996 (A N)' She wiIl hand over charge to Director, Health & Family Welfare Department. Haukhum Hauzel, Commisioner to the Govt. of Mizoram, Health & Family Welfare Department. \ 2 No. A.l1 013/1/94-EDN(L): the 31st October, 1996. In the interest of Public Service, the Governor of Mizoram is pleased to order tr:J.n<;f�r and posting of the fo llowing Lecturers to the Colleges shown against their nam�s with immediate effect. 51. Name of Lectura Present place New place Remarks No. of posting of posting I 2 3 4 5 • Pu T. -

3669 Ha Project Cost
0 AREA : 3669 Ha Project Cost : 550.35 Lakhs Hmawngbuchhuah, Kakichhuah, Sabualtlang. Prepared by, DO, Soil & Water Conservation Deptt. 1 INDEX CHAPTER Page No. 1. Introduction ------------------------------------------3 2. Project Profile ------------------------------------------8 3. Basic Information of Project Villages ------------------------------------------13 4. Participatory Rural Appraisal ------------------------------------------14 5. Problem Typology ------------------------------------------15 6. Project Intervention Plan ------------------------------------------18 7. DPR Plan Abstract ------------------------------------------19 8. Preparatory Phase. ------------------------------------------20 9. Work Plan Details ------------------------------------------21 10. Consolidation and Withdrawal Phase. ----------------------------------23 11. Capacity Building Institute Identified ----------------------------------24 12. Institutional & Capacity building Plan ----------------------------------25 13. Basic Profile of the project location -------- -------------------------27 14. Maps of the project ----------------------------------28 15. Institutional mechanism& Agreements. ----------------------------------32 16. SWOT Analysis of PIA. ----------------------------------33 17. PIA & Watershed Committee details. ----------------------------------34 18. Convergence Plans. ----------------------------------35 19. Expected Outcomes. ----------------------------------37 20. Expected Estimate Outcomes. ----------------------------------39 -

A Study of Correlation Between the Mnf And
© 2018 JETIR August 2018, Volume 5, Issue 8 www.jetir.org (ISSN-2349-5162) TRACING THE FLINCH OF INSURGENCY (A STUDY OF CORRELATION BETWEEN THE MNF AND ‘MAUTAM’ FAMINE IN MIZORAM) Dr Sakhawliana Assistant Professor, Department of Public Administration Government Kamalanagar College, Mizoram ABSTRACT Insurgency is causes, basically by political unwillingness of the administration. It is the call of the nations that the political track of different party’s should ensure nation building, and fixing the socio-economic menace of the general public. The prototyped imperialist, of premeditated coercive security and control of administration might not be viable to all sorts of establishments. Experience on the vital effects of the morale and psychology of the people can be seen in Mizoram, one of the North eastern states of India. This state has been under the profanity of insurgency for barely 20 years (1996-1986). It was said that after the famine cause by bamboo flowering, the philanthropic organisation of Mizo National Famine Front (MNFF) rechristened to form new political party of the Mizo National Front (MNF) and thrive for independence movement from the Indian Union. The hostility was brought by Mizo National Front (MNF) alias Mizo National Army (MNA), as its underground army wing, from one side and the Indian Army on the other. Over the year, the Indian Army and the MNA fought in tactical guerrilla warfare by using most sophisticated weapons of the 21st century. Therefore, it is impetus to unveil the insight of insurgency, in general, and the birth of the MNF for secession movement, in particular. -

F. No. Msdp-13/176/2017-Msdp-MOMA Government of India Ministry of Minority Affairs 11Th Floor, Pt Deen Dayal Antodaya Bhavan C.G.O
F. No. MsDP-13/176/2017-MsDP-MOMA Government of India Ministry of Minority Affairs 11th Floor, Pt Deen Dayal Antodaya Bhavan C.G.O. Complex, Lodi Road NewDelhi-110003 Dated: 27.09.2017 To, The Pay & Accounts Officer, Ministry of Minority Affairs, Paryavaran Bhavan, New Delhi Subject: Grant in aid under the Centrally Sponsored Scheme of Multi sectoral Development Programme for Minority Concentration District to Government of Mizoram for the year 2017-18 for Lawngtlai District. Sir, In continuation to this Ministry's sanction letter of even number dated 29.02.2016, I am directed to convey the sanction of the President for release of an amount of Rs 2, 91, 11,000/- (Rupees Two Crore Ninety-One Lakh Eleven Thousand Only) as 2nd instalment to the Govt. of Mizoram for implementing the scheme "Multi Sectoral Development Programme for Minority Concentration Districts" for Lawngtlai district as per the details enclosed at Annexure -I. The non-recurring grant may be released to the Govt. of Mizoramthrough CAS, Reserve Bank of India, Nagpur. 2. The State Government should ensure that proportionate share of State share for the projects mentioned at Annexure-I is released to the implementing agency along with Central share. 3. The expenditure is debitable to Demand No.66, Ministry of Minority Affairs Major Head- "3601" Grant-in-aid to State Governments, 06- Grants for State Plan Schemes (Sub Major Head), 101-General-(Welfare of Schedule Casts/Schedule Tribes and Other Backward Classes and Minorities) -Other Grants (Minor Head), 49 - Multi sectoral Development Programme for minorities, 49.00.35 - Grant for creation of capital assets for the year 2017-18. -
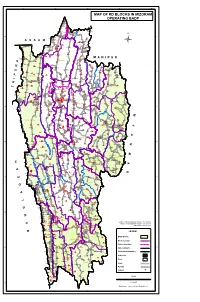
Map of Rd Blocks in Mizoram Operating Badp
92°20'0"E 92°40'0"E 93°60'0"E 93°20'0"E 93°40'0"E MAP OF RD BLOCKS IN MIZORAM Vairengte II OPERATING BADP Vairengte I Saihapui (V) Phainuam Chite Vakultui Saiphai Zokhawthiang North Chhimluang North Chawnpui Saipum Mauchar Phaisen Bilkhawthlir N 24°20'0"N 24°20'0"N Buhchang Bilkhawthlir S Chemphai North Thinglian Bukvannei I Tinghmun BuBkvIaLnKneHi IAI WTHLIR Parsenchhip Saihapui (K) Palsang Zohmun Builum Sakawrdai(Upper) Thinghlun(Lushaicherra) Hmaibiala Veng Rengtekawn Kanhmun South Chhimluang North Hlimen Khawpuar Lower Sakawrdai Luimawi KOLASIB N.Khawdungsei Vaitin Pangbalkawn Hriphaw Luakchhuah Thingsat Vervek E.Damdiai Bungthuam Bairabi New_Vervek Meidum North Thingdawl Thingthelh Lungsum Borai Saikhawthlir Rastali Dilzau H Thuampui(Zawlnuam) Suarhliap R Vengpuh i(Zawlnuam) i Chuhvel Sethawn a k DARLAWN g THINGDAWL Ratu n a Zamuang Kananthar L Bualpui Bukpui Zawlpui Damdiai Sunhluchhip Lungmawi Rengdil N.Khawlek Hortoki Sailutar Sihthiang R North Kawnpui I i R Daido a Vawngawnzo l Vanbawng v i Tlangkhang Kawnpui w u a T T v Mualvum North Chaltlang N.Serzawl i u u Chiahpui i N.E.Tlangnuam Khawkawn s T Darlawn a 24°60'0"N 24°60'0"N Lamherh R Kawrthah Khawlian Mimbung K Sarali North Sabual Sawleng Chilui Zanlawn N.E.Khawdungsei Saitlaw ZAWLNUAM Lungmuat Hrianghmun SuangpuilaPwnHULLEN Vengthar Tumpanglui Teikhang Venghlun Chhanchhuahna kepran Khamrang Tuidam Bazar Veng Nisapui MAMIT Phaizau Phuaibuang Liandophai(Bawngva) E.Phaileng Serkhan Luangpawn Mualkhang Darlak West Serzawl Pehlawn Zawngin Sotapa veng Sentlang T l Ngopa a Lungdai -

2017 Saikia Smitana 1218624
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ Explaining Divergent Outcomes of the Mizo and Bodo Conflicts in the Ethno-Federal Context of India’s Northeast Saikia, Smitana Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 30. Sep. 2021 King’s College London Explaining Divergent Outcomes of the Mizo and -

District Highlights 2011 Census
Census of India 2011 MIZORAM SERIES-16 PART XII-A DISTRICT CENSUS HANDBOOK LAWNGTLAI VILLAGE AND TOWN DIRECTORY DIRECTORATE OF CENSUS OPERATIONS MIZORAM i ii iii CENSUS OF INDIA 2011 MIZORAM SERIES-16 PART XII - A DISTRICT CENSUS HANDBOOK LAWNGTLAI VILLAGE AND TOWN DIRECTORY Directorate of Census Operations MIZORAM iv Motif PHAWNGPUI TLANG The highest peak in Mizoram with 2157 metres high near the Myanmar border in Lawngtlai district, the south-east region in Mizoram, considered to be the abode of the Gods. Phawngpui presents a fairy tale view of the blue hazed hills and vales unfolding below, the ridge stretches nearly 10 kms in north-south direction. Phawngpui or the blue mountain is situated near the state’s south-eastern border overlooking the bend of the mighty Chhimtuipui river and the hill ranges of Myanmar. The mountain area of Phawngpui at most. times is covered by a thin stretch of clouds, which make it blue in appearance from a distance, hence the name blue mountain. The temperature therefore is mild throughout the year ranging from 1.1-29°c, with aQ average rainfall of 3,000cm.The edges of the mountains are all very steep and mostly of sharp precipices of which Thlazuang Kham is the most spectacular one’. Abounding with diverse flora and fauna; Phawngpui was declared a National Park covering an area of about 50 sq km. It is famous for its exotic species of flora and fauna especially the orchids and rhododendrons spread within the valleys surrounding an all sides. The Pawngpui national park is the natural home ,of a wonderful range,of birdlife including the rare Blyth’s tragopan, falcan, sunbirds, dark-rumped swift and Mrs. -
(Aizawl-Tuipang) In
GOVERNMENT OF INDIA MINISTRY OF ROAD TRANSPORTATION AND HIGHWAYS NATIONAL HIGHWAYS AND INFRASTRUCTURE DEVELOPMENT CORPORATION GOVERNMENT OF INDIA PREPARATORY STUDY FOR NORTH EAST CONNECTIVITY IMPROVEMENT PROJECT IN INDIA WIDENING AND IMPROVEMENT OF NH54 (AIZAWL-TUIPANG) ENVIRONMENTAL IMPACT ASSESSMENT REPORT NOVEMBER 2015 JAPAN INTERNATIONAL COOPERATION AGENCY (JICA) NIPPON KOEI CO.,LTD. NIPPON KOEI INDIA PVD. LTD. 1 TABLE OF CONTENTS ABBRIVIATIONS ……………………………………………………………………………………………………………. iv EXECUTIVE SUMMARY……………………………………………………………………………………………….…… v Chapter 1 INTRODUCTION ........................................................................................................... 1 1.1 Background ......................................................................................................................... 1 1.2 Project Location .................................................................................................................. 1 1.3 Requirement and Objective of EIA Report ........................................................................... 2 1.4 Structure of EIA Report ........................................................................................................ 3 Chapter 2 DESCRIPTION OF PROJECT ......................................................................................... 5 2.1 Preparatory Study For North East Connectivity Improvement Project in India ..................... 5 2.2 Widening and Improvement of NH54 (Aizawl – Tuipang) ....................................................