A Case Study of Salford Quays
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PART 3 Scale 1: Publication Edition Scale 1: Publication Edition Scale 1: Publication Edition 44 W Nose of Howth to Ballyquintin Point 100,000 Oct
Natural Date of New Natural Date of New Natural Date of New Chart No. Title of Chart or Plan Chart No. Title of Chart or Plan Chart No. Title of Chart or Plan PART 3 Scale 1: Publication Edition Scale 1: Publication Edition Scale 1: Publication Edition 44 w Nose of Howth to Ballyquintin Point 100,000 Oct. 1978 Feb. 2001 1468w Arklow to the Skerries Islands 100,000 Aug. 1978 June 1999 1977w Holyhead to Great Ormes Head 75,000 Feb. 1977 Oct. 2001 105 w Cromer Knoll and the Outer Banks 75,000 Apr. 1974 Jan. 2010 1484w Plans in Cardigan Bay - Mar. 1985 Jan. 2002 1978w Great Ormes Head to Liverpool 75,000 Jan. 1977 May 2009 106 w Cromer to Smiths Knoll 75,000 Oct. 1974 Sept. 2010 A Aberystwyth 18,000 1981w Liverpool to Fleetwood including Approaches to Preston 75,000 Feb. 1977 May 2009 107 w Approaches to the River Humber 75,000 July 1975 May 2009 B Aberdovey 25,000 Preston Riversway Docklands 10,000 108 w Approaches to the Wash 75,000 June 1975 Apr. 2011 C Barmouth 25,000 2010wI Morecambe Bay and Approaches 50,000 Feb. 1988 July 2006 Wells-Next-The-Sea 30,000 D Fishguard Bay 15,000 2011w Holyhead Harbour 6,250 May 1975 Aug. 2005 109 wI River Humber and the Rivers Ouse and Trent 50,000 Dec. 1990 May 2009 E New Quay 12,500 2013w Saint Bees Head to Silloth 50,000 Feb. 1987 July 2010 A Humber Bridge to Whitton Ness 50,000 F Aberaeron 18,000 A Silloth Docks and Approaches 10,000 B3 B Whitton Ness to Goole and Keadby 50,000 G Newport Bay 37,500 B Maryport Harbour 10,000 C Keadby to Gainsborough 100,000 H Approaches to Cardigan 37,500 C Workington Harbour 7,500 D Goole 5,000 J Aberporth 30,000 D Harrington Harbour 10,000 111 w Berwick-upon-Tweed to the Farne Islands 35,000 July 1975 July 2009 1503wI Outer Dowsing to Smiths Knoll including Indefatigable Banks 150,000 Mar. -

History of the Manchester Ship Canal, from Its Inception to Its Completion
HISTORY OF THE MANCHESTER SHIP CANAL SIR BOSDIN LEECH to of tbe of Toronto lo. C . -CT : HISTORY OF THE MANCHESTER SHIP CANAL " Floreat Semper Mancunium DANIEL ADAMSON, FIRST CHAIRMAN OF THE MANCHESTER SHIP CANAL COMPANY. Elliott & Fry. Frontispiece. HISTORY OF THE MANCHESTER SHIP CANAL FROM ITS INCEPTION TO ITS COMPLETION WITH PERSONAL REMINISCENCES BY SIR BOSDIN LEECH NUMEROUS PLANS, PORTRAITS AND ILLUSTRATIONS IN TWO VOLUMES VOL I. 1*1 a s MANCHESTER AND LONDON: SHERRATT & HUGHES 1907 THE ABERDEEN UNIVERSITY PRESS LIMITED THESE VOLUMES ARE DEDICATED TO THE LORD MAYOR AND CORPORATION OF THE CITY OF MANCHESTER IN COMMEMORATION OF THE PUBLIC SPIRIT DISPLAYED BY THAT CITY IN COMING TO THE ASSISTANCE OF THE MANCHESTER SHIP CANAL AT A CRITICAL STATE OF ITS AFFAIRS, AND IN THE HOPE THAT THEIR EXAMPLE MAY STIMULATE FUTURE GENERATIONS TO SIMILAR LOCAL PATRIOTISM PREFACE. early struggles and ultimate triumph of the Manchester Ship Canal consti- THEtute a subject of absorbing interest. In the history of Manchester, and indeed of South Lancashire as a whole, no other event or enterprise can compare with it in its far-reaching effects. The story, too, in many respects contains all the elements of a romance. It is the relation of a desperate and almost hopeless fight against opposi- tion of the most powerful and uncompromising character, and it is meet that the names and qualities of the men engaged in the strife, and the nature of the difficulties which they encountered and overcame, should find a permanent record. To rescue both individuals and incidents from oblivion, and to give a connected narrative of the course of events from the conception to the completion of the canal, is the object of the present work. -

"Long Since, When All the Docks Were Filled with That Sea Beauty Man Has Ceased to Build" J.Masefield
LIVERPOOL N A U T I C A L RESEARCH SOCIETY "Long since, when all the docks were filled with that sea beauty man has ceased to build" J.Masefield ~IS, NOTES AND QUERIES Vol.XIII (New Series) No.1 January-March 1969 THE WRECK OF THE MISSOURI On the 17th February 1886, the screw steamer MISSOURI owned by the Warren Line cleared from Boston for Liverpool. She had been on this run for five years carrying cattle and general cargo to Liverpool, a trip which took about twelve days on average. A handsome iron built screw steamer, MISSOURI had come from the yard of James Connell on the Clyde in 1881. She had a length of 425ft., a gross tonnage of 3,332 and in addition to her engines she had a four masted barque rig. On this particular voyage she carried 395 head of cattle and 4,000 tons of general cargo, comprising bales of cotton, sacked flour, palm oil tallow, and a large quantity of provisions. In addition to the crew of 64, there were 18 cattlemen to look after the beasts. At a later date it transpired that there were also three stowaways aboard. The Master of the MISSOURI, Captain Poland had commanded the vessel since she came into service. Before that he had spent six years in command of sailing vessels in the Indian. Ocean. The Company had a very high regard of his capabilities. - 1 - By the 28th February MISSOURI had reached the Irish Sea, and at 8 p.m. was ~ miles from the 'fuskar Rock with a gale blowing. -

NW Europe Standard Nautical Charts and Leisure Products Catalogue 2020 Tek Marine Ltd
A selection of UK & Republic of Ireland DISTRIBUTORS: Supplying Standard Paper Nautical ADMIRALTY Charts and Publications (P) and digital products (D) and have print on demand (POD) capability. ADMIRALTY Location Distributor Telephone Website Type Leisure Folios Aberdeen Global Navigation Solutions Ltd. +44 (0)1224 595 045 www.gnsworldwide.com P, D & POD Aberdeen Nautisk + 44 (0)1224 959850 www.nautisk.com P, D & POD ADMIRALTY Leisure produces a range of leisure Aberdeen PiSys Marine Ltd. +44 (0)1651 277 000 www.pisysmarine.com D folios specifi cally designed for the leisure user. Bangor Todd Navigation +44 (0)2891 466 640 www.toddchart.com P, D & POD Bristol Nautisk +44 (0)1454 617 636 www.nautisk.com P, D & POD SC5616 Buckie Poseidon Navigation Services Ltd. +44 (0)1542 841 245 www.poseidonnavigation.com P, D & POD Chichester Da Gama Maritime Ltd. +44 (0)1243 511 084 www.dgmaritime.com P, D & POD Enfield ChartCo Ltd. +44 (0)1992 805 400 www.chartco.com P, D & POD SC5617 Fareham Euronav Ltd. +44 (0)2392 373 855 www.euronav.co.uk D Grimsby South Bank Marine Charts Ltd. +44 (0)1472 361 137 www.southbankmarine.com P, D & POD Hull B. Cooke & Son Ltd. +44 (0)1482 223 454 www.bcookeandson.co.uk P, D & POD Ivybridge PC Maritime Ltd. +44 (0)1752 254 205 www.pcmaritime.com D London Stanfords Maritime Books and Charts +44 (0)207 759 7150 www.stanfords.com P, D & POD Charity & Taylor Lowestoft +44 (0)1502 573943 www.charityandtaylor.com P, D & POD (Charts & Publications) Ltd. -

Liverpool, Manchester and Market Power: the Ship Canal and the North West Business Landscape in the Late Nineteenth Century
Liverpool, Manchester and market power: The Ship Canal and the North West business landscape in the late nineteenth century Graeme J. Milne The grooves in which trade runs are exceeding deep, and it needs an infinite amount of trouble to make it face the jolt and effort of the change even when the new groove is demonstrably far better than the old. The opening of the Manchester Ship Canal in 1894 was an important event in the history of late Victorian engineering, trade and transport, and its construction and early operations have spawned a considerable body of literature.2 Some of the canal’s wider and longer-term implications are less well known, however. The Ship Canal raised major questions of civic pride and identity, becoming a shibboleth for ‘patriotic’ Liverpudlians and Mancunians, and challenged assumptions about the relative power and influence of seaports and inland, manufacturing cities. This article focuses on Manchester’s attempts to expand its trade after the opening of the canal, and the extent to which it was thwarted by obstruction from Liverpool and by the inertia of established trading systems. As such, it explores economic and business aspects of a broader regional controversy that have perhaps been studied less fully than earlier disputes about the actual building of the canal.J 1 Manchester Guardian, 29 August 1894. 2 For example, Bosdin Leech, History o f the Manchester Ship Canal from its inception to its completion (Manchester, 1907); David Owen, The Manchester Ship Canal (Manchester, 1983). 3 Important exceptions are D.A. Famie, The Manchester Ship Canal and the rise o f the port o f Manchester, 1874-1975 (Manchester, 1980); Ian Harford, Manchester and its ship canal movement: Class, work and politics in late- 126 Graeme J. -

They Knew Why They Fought
THEY KNEW WHY THEY FOUGHT UNOFFICIAL STRUGGLES & LEADERSHIP ON THE DOCKS 1945-1989 Bill Hunter © Copyright 1994 Bill Hunter Published by Index Books (Indexreach) Ltd. 28 Charlotte Street, London W1P 1HJ Typeset by Art in Action 051 933 5168 Printed by Trade Union Printing Services, Newcastle Upon Tyne (re-printed by Living History Library in 2008) A C.I.P. catalogue record for this book is available from the British Library ISBN 1-871518-09-1 Dedication In memory of Rachel Ryan 1917 - 1992 My companion and comrade for forty eight years and a Trotskyist for fifty four. With the best of the unofficial leaders who appear in this book: "She knew why she fought, and loved what she knew." 1 Illustrations in original book Cover: Dockers Demonstration 1952 Building The Union 1: Blue Union Membership Book Authors Collection 2: Peter Kerrigan Art in Action 3: Gerry Edwards Art in Action 4: 1951 Conspiracy Trial The Newsletter 5: Socialist Outlook Authors Collection 6: Liverpool Portworkers Committee 1952 Building The Union 7: Portworkers Clarion Authors Collection 8: News of the Blues Authors Collection 9: What Next For Britain's Portworkers Authors Collection 10: Pay Dispute 1970 Marg Nichols 11: Jack Jones is questioned by dockers The Newsletter 12: Release of Vic Turner 1974 Marg Nichols 13: Liverpool Docks 1920 Merseyside County Museums 14: Liverpool Docks 1980 Art in Action Back: The Author Art in Action Contents 1: Struggling Out of the Abyss. 2: The Desire for Change. After the War 3: The Unofficial Movements 4: The T&GWU and the Break to the Blue Union 5: The Blue Union Recognition Strike 6: Rank and File Unity in defence of the "Blue" 7: The "Decasualisation" Deals 8: The Devlin Scheme 9: The 1967 Strike 10: Modernisation 11: Jack Jones and the Jones-Aldington Committee 12: The Betrayal of the Anti-Tory Struggles 13: Abolition of the Scheme - The Final Sell-Out 14: Balance Sheet of the Blue 15: Conclusions 2 Preface This book does not have its origins in academic considerations of writing history. -

Manchester by the Sea
Trail Manchester by the sea Explore the stories behind the headlines of Salford’s media city © Rory Walsh Time: 1½ hours Distance: 1½ miles Landscape: urban Coronation Street and Countdown… Match of Location: the Day and In the Night Garden... Blue Peter Salford Quays, Salford, Greater Manchester and The Jeremy Kyle Show… welcome to one of Britain’s starriest places. Start: The Plaza, BBC MediaCityUK, M50 2EQ Some 35,000 people live or work at Salford Quays. This gleaming complex in Greater Finish: Manchester includes houses, museums, Centenary Walkway, Salford Wharf galleries, and most famously, television and radio studios. Grid reference: SJ 80274 97308 Yet at the turn of the millennium this was an empty brownfield site, rescued from the Be prepared: remains of derelict docks built nearly 40 miles Take care of young children by the water inland. Keep an eye out for: From ghost trains and banana boats to race Daleks, Pudsey Bear, Upsy Daisy, and various horses and wild deer, hear the stories behind sculptures and artworks along the paths the headlines of this modern media landmark. Directions The walk starts at The Plaza, the large public square next to the MediaCityUK Metrolink stop. To begin, go into the centre of The Plaza and face the BBC Studios. Every landscape has a story to tell – find out more at www.discoveringbritain.org Route and stopping points 01 The Plaza, BBC MediaCityUK 05 NV Buildings 02 MediaCityUK Bridge 06 Detroit Bridge 03 Imperial War Museum North 07 Ontario Basin 04 The Lowry 08 Centenary Walkway Every landscape has a story to tell – Find out more at www.discoveringbritain.org 01 The Plaza, BBC MediaCityUK We begin the walk with a sight that may seem familiar. -

Should We Move Public Sector Jobs out of London? August 2017
Should we move public sector jobs out of London? August 2017 The relocation of public sector jobs is one of the most direct tools that policy makers can use to move jobs around the country. There is precedent for doing this already in the UK – for example through the opening of HM Revenue and Customs offices in Liverpool, Department for Work and Pensions in Newcastle and the Driver and Vehicle Licensing Agency in Swansea. And political interest continues on this front. As part of its industrial strategy and rebalancing agenda, the 2017 Conservative Party manifesto pledged to move significant numbers of civil servants out of London and the South East to other cities in the UK. Governments often use relocation of public sector workers to try and stimulate economic growth in different parts of the country. Public sector relocation can stimulate the local economy in two main ways. The first, direct impact is the move of the jobs themselves and the wages they pay. The second is the ‘multiplier effect’ that these jobs can have, boosting demand for local goods and services and attracting jobs in related industries by improving the attractiveness of the area to businesses. The size of the multiplier effect on the local area is affected by the skills levels and nature of the jobs as well as the number. For example, the movement of low-skilled jobs has a clear direct impact in that it creates employment. But its wider impact is likely to be limited, in that the wages they pay will be lower, the career progression they offer is likely to be limited and their interaction with other bodies, be they public or private (e.g. -

Pre-Employment Questionnaire
19 January 2021 Mr Brian Sims Planning Inspector c/o Ms Elizabeth Humphrey The Planning Inspectorate Temple Quay House 2 The Square Bristol BS1 6PN Dear Mr Sims, HAYOCK POINT APPEAL, ST HELENS REF. APP/H4315/W/20/3256871 I am writing as Chief Executive of the Peel Group of companies, to confirm the role of the different companies involved in the Haydock Point project that carry the Peel name, and to outline our approach to the Haydock Point scheme. Company roles The Peel Group is an entity with a number of listed and unlisted investments across the real estate and infrastructure sectors, and is one of the country’s largest such private investors with collective investments owned and under management of over £5 billion. The Peel Group is the parent company to Peel L&P and also has major shareholdings in Peel Ports Group, PLP (Peel Logistics Property) and Harworth Group, amongst others. Our key investment sectors are real estate, transport and logistics, low-carbon energy and infrastructure. Peel L&P is the core direct land and property operating business wholly owned by Peel Group. It has a diverse land portfolio including sites for strategic development such as the Haydock Point site which the company has held for over 30 years. Peel L&P is the applicant, landowner and promoter of the Haydock Point scheme. PLP (Peel Logistics Property) is the specialist “big box” logistics developer owned by Peel Group, Ivanhoe Cambridge, MIRA Real Estate and the PLP senior management team. PLP has a very strong UK wide track record in delivering, owning and managing large scale developments such as Haydock Point. -

A Sheffield Hallam University Thesis
Railways, land-use planning and urban development : 1948-94. HAYWOOD, Russell. Available from the Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/19777/ A Sheffield Hallam University thesis This thesis is protected by copyright which belongs to the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Please visit http://shura.shu.ac.uk/19777/ and http://shura.shu.ac.uk/information.html for further details about copyright and re-use permissions. Fines are charged at 50p per hour 2 4 SEP 2003 H- I fp M Z\ 2 1 NOV^OP ProQuest Number: 10697079 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10697079 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Railways, Land-Use Planning and Urban Development: 1948-94 Russell Haywood A thesis submitted in partial fulfilment of the requirements of Sheffield Hallam University for the degree of Doctor of Philosophy January 2001 Thanks Many people and organisations have provided me with help and support over the five years or so that I have been carrying out this research. -

Working Paper Manchester / Liverpool
Shr ink ing Cit ies -------------------------------------------------------------------------------- MANCHESTER / LIVERPOOL II LIVERPOOL / MANCHESTER ARBEITSMATERIALIEN WORK ING PAPERS PABOCIE MATEPIALY ------------------------------------------------------------------------------------ MANCHESTER / LIVERPOOL MANCHESTER / LIVERPOOL II --------------------------------------------- March 2004 -------------------------------------------------------------------------------------------------------------- Shrinking Cities A project initiated by the Kulturstiftung des Bundes (Federal Cultural Foundation, Germany) in cooperation with the Gallery for Contemporary Art Leipzig, Bauhaus Foundation Dessau and the journal Archplus. Office Philipp Oswalt, Eisenacher Str. 74, D-10823 Berlin, P: +49 (0)30 81 82 19-11, F: +49 (0)30 81 82 19-12, II [email protected], www.shrinkingcities.com TABLE OF CONTENTS -------------------------------------------------------------------------------------------------------------- 3INTRODUCTION Philipp Misselwitz 5MAP OF THE M62 REGION 6STATISTICAL DATA: MANCHESTER / LIVERPOOL Ed Ferrari, Anke Hagemann, Peter Lee, Nora Müller and Jonathan Roberts M62 REGION 11 ABANDONMENT AS OPPORTUNITY Katherine Mumford and Anne Power 17 CHANGING HOUSING MARKETS AND URBAN REGENERATION: THE CASE OF THE M62 CORRIDOR Brendan Nevin, Peter Lee, Lisa Goodson, Alan Murie, Jenny Phillimore and Jonathan Roberts 22 CHANGING EMPLOYMENT GEOGRAPHY IN ENGLAND’S NORTH WEST Cecilia Wong, Mark Baker and Nick Gallent MANCHESTER 32 MANCHESTER CITY -
Generated by Foxit PDF Creator © Foxit Software for Evaluation Only
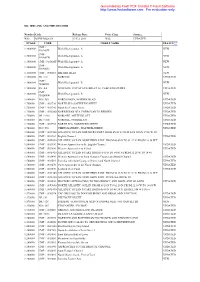
Generated by Foxit PDF Creator © Foxit Software http://www.foxitsoftware.com For evaluation only. UK, IRELAND AND THE CHANNEL Number Code Release Date Price Class Status * W88 M-EW-M226.04 17 Feb 2011 Wide UPDATED SCALE CODE CHART NAME STATUS ** CMP / 1:3000000 World Background - A NEW 910A05N CMP / 1:3000000 World Background - A NEW 910A04W 1:3000000 CMP / 910A06F World Background - A NEW CMP / 1:3000000 World Background - A NEW 910A05O 1:1500000 CMP / 1000310 BRITISH ISLES NEW 1:1500000 DE / 101 NORDSEE UPDATED CMP / 1:1000000 World Background - B NEW 910B05N 1:1000000 ES / 4A GOLFO DE VIZCAYA DE BREST AL CABO FINISTERRE UPDATED CMP / 1:1000000 World Background - B NEW 910B04W 1:800000 NO / 559 NORDSJOEEN, NORDRE BLAD UPDATED 1:750000 CMP / 1003720 NORTH SEA SOUTHERN SHEET UPDATED 1:750000 CMP / 1003740 North Sea Central Sheet UPDATED 1:750000 CMP / 100V800 NORWEGIAN SEA, FOEROYAR TO BERGEN UPDATED 1:750000 DE / 1001 NORDSEE, MITTELBLATT UPDATED 1:750000 DE / 1000 NORDSEE, NORDBLATT UPDATED 1:750000 CMP / 1003730 NORTH SEA, NORTHERN SHEET UPDATED 1:700000 NO / 558 VIKINGBANKEN - HALTENBANKEN UPDATED 1:500000 CMP / 100Y500 ATLANTIC OCEAN NORTHERN PART FROM 45 00 N TO 48 24 N FRON 19 06 W TO 1:500000 CMP / 1001220 English Channel UPDATED 1:500000 CMP / 100Y450 ATLANTIC OCEAN. NORTHERN PART. FROM 48 00 N TO 51 12 N. FROM 18 58 W T 1:500000 CMP / 1003190 Western Approaches to the English Channel UPDATED 1:500000 CMP / 1003480 Western Approaches to Ireland UPDATED 1:500000 CMP / 100Y410 ATLANTIC OCEAN N PART FROM 51 00 N TO 54 00 N FROM 21 26 W TO 14 40 1:500000 CMP / 1003490 Western Approaches to Saint George's Channel and Bristol Channel UPDATED 1:500000 CMP / 1003510 Irish Sea with Saint George's Channel and North Channel UPDATED 1:500000 CMP / 1003470 Outer Approaches to the North Channel UPDATED 1:500000 CMP / 1003570 Scotland West Coast UPDATED 1:500000 CMP / 100Y360 ATLANTIC OCEAN.