Micropropagation of Two Species of Micranthocereus (Cactaceae) with Ornamental Potential Native to Bahia, Brazil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae) Lendel, Anita Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-93287 Dissertation Published Version Originally published at: Lendel, Anita. South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). 2013, University of Zurich, Faculty of Science. South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae) _________________________________________________________________________________ Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr.sc.nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Anita Lendel aus Kroatien Promotionskomitee: Prof. Dr. H. Peter Linder (Vorsitz) PD. Dr. Reto Nyffeler Prof. Dr. Elena Conti Zürich, 2013 Table of Contents Acknowledgments 1 Introduction 3 Chapter 1. Phylogenetics and taxonomy of the tribe Cereeae s.l., with particular focus 15 on the subtribe Trichocereinae (Cactaceae – Cactoideae) Chapter 2. Floral evolution in the South American tribe Cereeae s.l. (Cactaceae: 53 Cactoideae): Pollination syndromes in a comparative phylogenetic context Chapter 3. Contemporaneous and recent radiations of the world’s major succulent 86 plant lineages Chapter 4. Tackling the molecular dating paradox: underestimated pitfalls and best 121 strategies when fossils are scarce Outlook and Future Research 207 Curriculum Vitae 209 Summary 211 Zusammenfassung 213 Acknowledgments I really believe that no one can go through the process of doing a PhD and come out without being changed at a very profound level. -

CACSS Seed Depot
CACSS Seed Depot Sold for $1 per packet to members by the Propagation Education Group (PEG) Genus Species Common Name ID Adenium 142 Seed Origin Collection/ Collector's # Purity Date Collected Unknown Quantity Packets Available Dave's Garden Link 1 Genus Species Common Name ID Adenium hybrid - double white Desert Rose 3 Seed Origin Collection/ Collector's # Purity Date Collected Quantity Packets Available Dave's Garden Link 2 Genus Species Common Name ID Adenium Thai soco 141 Seed Origin Collection/ Collector's # Purity Date Collected Unknown Quantity Packets Available Dave's Garden Link 1 Genus Species Common Name ID Aeonium urbicum Saucer Plant 133 Seed Origin Collection/ Collector's # Purity Date Collected Open-Pollinated 8/28/16 Quantity Packets Available Dave's Garden Link 8 http://davesgarden.com/guides/pf/go/55105/ Genus Species Common Name ID Agave colimana 170 Seed Origin Collection/ Collector's # Purity Date Collected Chandler, AZ Open-Pollinated 2019 Quantity Packets Available Dave's Garden Link 11 Sunday, March 8, 2020 Page 1 of 14 Genus Species Common Name ID Aloe harlana 131 Seed Origin Collection/ Collector's # Purity Date Collected Unknown 5/2016 Quantity Packets Available Dave's Garden Link 1 http://davesgarden.com/guides/pf/go/58446/ Genus Species Common Name ID Aloe humilis 130 Seed Origin Collection/ Collector's # Purity Date Collected Unknown 6/2016 Quantity Packets Available Dave's Garden Link 3 Genus Species Common Name ID Asclepias curassavica Tropical Milkweed 59 Seed Origin Collection/ Collector's # Purity Date -

Phylogenetic Relationships in the Cactus Family (Cactaceae) Based on Evidence from Trnk/Matk and Trnl-Trnf Sequences
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/51215925 Phylogenetic relationships in the cactus family (Cactaceae) based on evidence from trnK/matK and trnL-trnF sequences ARTICLE in AMERICAN JOURNAL OF BOTANY · FEBRUARY 2002 Impact Factor: 2.46 · DOI: 10.3732/ajb.89.2.312 · Source: PubMed CITATIONS DOWNLOADS VIEWS 115 180 188 1 AUTHOR: Reto Nyffeler University of Zurich 31 PUBLICATIONS 712 CITATIONS SEE PROFILE Available from: Reto Nyffeler Retrieved on: 15 September 2015 American Journal of Botany 89(2): 312±326. 2002. PHYLOGENETIC RELATIONSHIPS IN THE CACTUS FAMILY (CACTACEAE) BASED ON EVIDENCE FROM TRNK/ MATK AND TRNL-TRNF SEQUENCES1 RETO NYFFELER2 Department of Organismic and Evolutionary Biology, Harvard University Herbaria, 22 Divinity Avenue, Cambridge, Massachusetts 02138 USA Cacti are a large and diverse group of stem succulents predominantly occurring in warm and arid North and South America. Chloroplast DNA sequences of the trnK intron, including the matK gene, were sequenced for 70 ingroup taxa and two outgroups from the Portulacaceae. In order to improve resolution in three major groups of Cactoideae, trnL-trnF sequences from members of these clades were added to a combined analysis. The three exemplars of Pereskia did not form a monophyletic group but a basal grade. The well-supported subfamilies Cactoideae and Opuntioideae and the genus Maihuenia formed a weakly supported clade sister to Pereskia. The parsimony analysis supported a sister group relationship of Maihuenia and Opuntioideae, although the likelihood analysis did not. Blossfeldia, a monotypic genus of morphologically modi®ed and ecologically specialized cacti, was identi®ed as the sister group to all other Cactoideae. -

Feb 09 Communique.Indd
San Gabriel Valley Cactus & Succulent Society COMMUNIQUE An Affi liate of the Cactus & Succulent Society of America, Inc. February 2009 - Volume 42, Number 2 February Meeting: President’s Message Thursday, I hope that everyone who could, came out to enjoy that glorious February 12 at 7:30 pm day at the Huntington. Perfect weather, succulents in bloom, fantastic. I for one appreciate the efforts that the staff and volunteers (several SGVCSS members included) of the Botanical Gardens expend for Meetings are held on the those of us in the C&S hobby. 2nd Thursday of the month Mark your calendar - July 18 is the date for our trip to Lotus Land. at 7:30 pm in the Palm Room, More details will be forthcoming. Los Angeles County Arboretum, We attended the Haworthia Hoedown last night, excellent turn- Arcadia. out. Thanks Patty and Rene Caro for adding a little spice to the Study Group. The plants that members brought to share and to trade were great. Mini-Show Plants: We hear that Jim Hanna is on the mend after 15 days in the CACTUS: (continued on page 3) Ceroids This Month’s Program SUCCULENT: Gasteria Our speaker for February will be Tim Nomer. Tim is very famil- iar for those who attend any of the C&S shows in the area. He and his wife, Anat are seen at all of the shows photographing the plants. He Study Group: will present a digital slide show that will highlight recent San Gabriel Study group will meet on Wednesday, and Intercity shows. His interesting perspectives on what makes a February 18th in the Palm Room, plant worthy of a second look give us all a hint at how better to prepare Los Angeles County Arboretum at our plants for shows. -

RMB-439 C-Formato.Indd
Revista Mexicana de Biodiversidad 81: 163- 175, 2010 http://dx.doi.org/10.22201/ib.20078706e.2010.001.186 Is geographical rarity frequent among the cacti of the Chihuahuan Desert? ¿Es la rareza geográfi ca frecuente entre las cactáceas del Desierto Chihuahuense? Héctor M. Hernández*, Carlos Gómez-Hinostrosa and Gibrán Hoffmann Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70-233, 04510 Mexico D. F., Mexico. *Correspondent: [email protected] Abstract. With the aim of assessing the extent of geographical rarity of Mexican Cactaceae, we calculated the distribution size (area of occupancy) of 142 species from the Chihuahuan Desert. In addition, using 2 variables (number of localities and range size), we preliminarily assessed their conservation status using the current IUCN Red List criteria. The results showed enormous variation in the areas of occupancy, although from the biogeographic and conservation perspective the most exceptional group comprises the extremely narrow endemics (42 species), whose range is restricted to areas smaller than 10 km2. Our results reinforce the reputation of this plant family as exceptionally rare geographically. We suggest that geographical rarity of Cactaceae in the Chihuahuan Desert is a natural phenomenon; however, we propose that the range of several species has been infl uenced by human activities. Regarding the conservation status of the species, 75 of them are categorized as Least concern. The remaining 67 species (47.2%) fall in 1 of the 3 categories of threat (27 Vulnerable, 11 Endangered, and 29 Critically endangered). These fi gures confi rm the critical conservation status of Mexican Cactaceae. -

Cactus Explorers Journal
Bradleya 34/2016 pages 100–124 What is a cephalium? Root Gorelick Department of Biology and School of Mathematics & Statistics and Institute of Interdisciplinary Studies, Carleton University, 1125 Raven Road, Ottawa, Ontario K1S 5B6 Canada (e-mail: [email protected]) Photographs by the author unless otherwise stated. Summary : There are problems with previous at - gibt meist einen abgrenzbaren Übergang vom tempts to define ‘cephalium’, such as via produc - photosynthetisch aktiven Gewebe zum nicht pho - tion of more hairs and spines, confluence of tosynthetisch aktiven und blütentragenden areoles, or periderm development at or under - Cephalium, die beide vom gleichen Triebspitzen - neath each areole after flowering. I propose using meristem abstammen. Cephalien haben eine an - the term ‘cephalium’ only for a combination of dere Phyllotaxis als die vegetativen these criteria, i.e. flowering parts of cacti that Sprossabschnitte und sitzen der vorhandenen have confluent hairy or spiny areoles exterior to a vegetativen Phyllotaxis auf. Wenn blühende Ab - thick periderm, where these hairs, spines, and schnitte nur einen Teil der oben genannten Merk - periderms arise almost immediately below the male aufweisen, schlage ich vor, diese Strukturen shoot apical meristem, and with more hairs and als „Pseudocephalien“ zu bezeichnen. spines on reproductive parts than on photosyn - thetic parts of the shoot. Periderm development Introduction and confluent areoles preclude photosynthesis of Most cacti (Cactaceae) are peculiar plants, cephalia, which therefore lack or mostly lack even for angiosperms, with highly succulent stomata. There is almost always a discrete tran - stems, numerous highly lignified leaves aka sition from photosynthetic vegetative tissues to a spines, lack of functional photosynthetic leaves, non-photosynthetic flower-bearing cephalium, CAM photosynthesis, huge sunken shoot apical both of which arise from the same shoot apical meristems, and fantastic stem architectures meristem. -

Bradleya 31/2013 Pages 142-149
Bradleya 31/2013 pages 142-149 Coleocephalocereus purpureus has a cephalium; Micrantho - cereus streckeri has a pseudocephalium (Cereeae, Cactoideae, Cactaceae) Root Gorelick Department of Biology, School of Mathematics & Statistics, and Institute of Interdisciplinary Studies Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6 Canada. (email: [email protected]). Photographs by the author Summary : The putatively closely related cactus Introduction genera of Coleocephalocereus , Micranthocereus , Cactaceae (cacti) in the tribe Cactoideae have Cereus , Monvillea , and Stetsonia have a wide a wide range of reproductive anatomies ranging range in specialization of reproductive portions of from cephalia to pseudocephalia to forms where the shoot, from cephalium to pseudocephalium to reproductive and vegetative structures are indis - no specialization. After briefly summarizing the tinguishable (Buxbaum, 1964, 1975; Mauseth, shifting uses of the terms ‘cephalium’ and ‘pseudo - 2006). cephalium’, I provide gross morphological evi - For instance, Melocactus Link & Otto, Disco - dence that Coleocephalocereus purpureus has a cactus Pfeiffer, and Espostoa Britton & Rose have true cephalium that is formed of a continuous true cephalia in which the flowering parts are not swath of bristles and hairs, with its underlying photosynthetic because every epidermal cell con - thick cortex of parenchyma replaced by a narrow tains a modified leaf that is a hair, bristle, or layer of cork. By contrast, Micranthocereus streck - spine, with no stomata amongst the epidermal eri has a pseudocephalium composed of nothing cells (Mauseth, 2006). Furthermore, there are more than larger hairier areoles in which the un - changes to the internal anatomy of cephalia, derlying epidermis is still photosynthetic and the where the underlying cortex is not a wide swath of underlying cortex is still a thick layer of highly succulent parenchyma, but instead a thin parenchyma without any noticeable cork. -
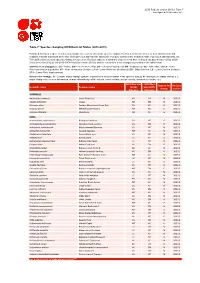
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

In Vitro Mass Micropropagation of Mammillaria Vetula Ssp. Gracilis Var
In Vitro Mass Micropropagation of Mammillaria Vetula ssp. Gracilis var. Arizonica Snowcap Manuel Lopez-Granero, M., López Granero IHSM: Instituto de Hortofruticultura Subtropical y Mediterranea Antonio Arana IHSM: Instituto de Hortofruticultura Subtropical y Mediterranea Jose Javier Regalado González ( [email protected] ) Consejo Nacional de Investigaciones Cienticas y Tecnicas https://orcid.org/0000-0003-1664-2000 Carlos Lopez Encina IHSM: Instituto de Hortofruticultura Subtropical y Mediterranea Research Article Keywords: Cactus, propagation in vitro, regeneration, ornamental, rooting. Posted Date: August 3rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-738336/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/21 Abstract A mass micropropagation method was developed for Mammillaria vetula ssp. gracilis cv. Arizonica Snowcap, a cactus species with high ornamental value. A culture media based in MS medium (Murashige − 1 − 1 − 1 − 1 and Skoog, 1962) supplemented with 0.1 mg l NAA + 0.3 mg l KIN + 5.0 mg l GA3 + 30 g l sucrose (MSM-1), was developed specically for the establishment and multiplication of Mammillaria vetula cv. Snowcap offshoots, let us to produce a proliferation rate of 34.9 ± 5.9 new offshoots per explant. The non-rooted offshoots were rooted in MSM-2, consisting in MS salts supplemented with 0.1 mg l− 1 NAA + 0.3 mg l− 1 KIN plus 5 mg l− 1 ancymidol and a lower dose (10 g l− 1) of sucrose, with a rooting rate of 100%. The rate of acclimatization of the rooted offshoots was 100%. -

Espostoa(Vatricania)
Haseltonia58 26: 58–67. 2019 Haseltonia 26: 58–67. 201958 Espostoa (Vatricania) guentheri have unusual cephalia or pseudocephalia Root Gorelick Department of Biology and School of Mathematics & Statistics and Institute of Interdisciplinary Stud- ies Carleton University, 1125 Raven Road (unceded Algonquin territory),Ottawa, Ontario K1S 5B6 Canada. Email: [email protected] Manuscript received 19th August 2019 Abstract: Espostoa (Vatricania) guentheri is a peculiar putative Espostoa, having some traits in common with Espostoa sensu stricto (cephalia in which many or most epidermal cells produces a spine, bristle, or trichome), some traits in common with Thrixanthocereus (cephalia with bristles instead of hairs; patches of epidermal cells in the cephalium that lack spines, bristles, hairs or trichomes), and some unique traits (gradual transition to cephalium formation; disjunct habitat). Gradual cephalium formation in E. guentheri is common, but not universal, which might be either because Vatricania is not closely related to Espostoa or that Vatricania is of hybrid origin. E. guentheri may have a pseudocephalium, rather than a true cephalium, with the reproductive part of shoots in subgenus Vatricania being morphologically intermediate between those of subgenus Espostoa and subgenus Thrixanthocereus. Keywords: cephalium, Espostoa, disjunct species, hybrid origin, pseudocephalium, Thrixanthocereus, Vatricania INTRODUCTION Previously (Gorelick, 2016: 118), I defined a cephalium as: part of a cactus shoot arising directly from the shoot apical meristem, with the cephalium composed of confluent areoles from which flow- ers originate, bearing copious spines and trichomes, and underlain by a thick periderm in lieu of an even thicker cortex. Bristles and hairs on flowering parts are longer than those on non-flowering parts. -

Volume 6 Number 22 1'
I ll VOLUME 6 NUMBER 22 1' DENMOZA ERYTHROCEPHALA Photograph & Collection - Mrs L.E. McIntosh Denmoza erythrocephala SEED Denmoza rhodocantha SEED DENMOZA ERYTHROCEPHALA FLOWERS from Mrs L.E.McIntosh This plant was grown from seed which I purchased from Winter in 1957 and it flowered for the first time at the age of ten years whilst still globular. The plant has elongated over the last five years and is now 8" tall and 5" across (not Including the spines). It is an attractive plant with a brownish green body, many large pink wool Iy areoles, heavy spines which are deep red when new, passing to rust red and fin a lly to grey at the bottom of the p la n t. When globular it had several silky white hairlike bristles about 1" long from all the areoles but it has now lost these from the old growth and only retains them in the top third of the plant. The buds appear in early summer as smalI balls of silky white hairs from the top of the areoles in last season's growth. They take approximately four weeks to grow to maturity and are then about 2" tall, tightly closed at the top and slightly zygomorphic, bright purple in colour with very fleshy shiny scales about 5/8" long, symmetrically arranged and covering the tube; tufts of white silky hairs grow out from under the scales. The top of the flower then opens to reveal a row of petals, slightly longer than the scales, lighter in colour with an orange overtone and much finer in texture, from which protrudes a large pinky cream stigma with 8 lobes surrounded by a tightly packed bunch of stamens of the same colour as the petals, the colour paling to almost white at the base of the filaments and shading out to deep purple anthers topped with purple pollen. -

Redalyc.Is Geographical Rarity Frequent Among the Cacti of the Chihuahuan Desert?
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Hernández, Héctor M.; Gómez-Hinostrosa, Carlos; Hoffmann, Gibrán Is geographical rarity frequent among the cacti of the Chihuahuan Desert? Revista Mexicana de Biodiversidad, vol. 81, núm. 1, abril, 2010, pp. 163-175 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42515998021 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 81: 163- 175, 2010 Is geographical rarity frequent among the cacti of the Chihuahuan Desert? ¿Es la rareza geográfi ca frecuente entre las cactáceas del Desierto Chihuahuense? Héctor M. Hernández*, Carlos Gómez-Hinostrosa and Gibrán Hoffmann Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70-233, 04510 Mexico D. F., Mexico. *Correspondent: [email protected] Abstract. With the aim of assessing the extent of geographical rarity of Mexican Cactaceae, we calculated the distribution size (area of occupancy) of 142 species from the Chihuahuan Desert. In addition, using 2 variables (number of localities and range size), we preliminarily assessed their conservation status using the current IUCN Red List criteria. The results showed enormous variation in the areas of occupancy, although from the biogeographic and conservation perspective the most exceptional group comprises the extremely narrow endemics (42 species), whose range is restricted to areas smaller than 10 km2.